![Lipid & it's metabolism [Part 2] (Viva)](https://emedicodiary.com/images/queimg/497f318b0afb7c17ef3b6ba75839b9a8.jpg)
Lipid & it's metabolism [Part 2] (Viva)
Q.70 Name the substrates required in adipose tissue for TG synthesis.
Two substances are required:
- α-glycero-P and
- Acyl CoA-activated FFA
Q.71 Name the enzymes required for the breakdown of TG (lipolysis).
Three enzymes are required:
Triacyl glycerol lipase-Hormone sensitive and key rate-limiting enzyme. The enzyme can exist in “active” or “inactive” state.
- Diacyl glycerol lipase
- Monoacyl glycerol lipase
Both are non-hormone sensitive.
Q.72 How the action of TG lipase is regulated?
- TG lipase can exist in active “a” and inactive “b” forms.
- Increased cyclic AMP↑ level in the cells converts “inactive” cyclic AMP-dependent protein kinase (C2R2) to “active” protein kinase (C2), which in turn phosphorylates “inactive” hormone-sensitive TG lipase “b” to “active” TG lipase “a” which breaks down TG to form DG + FFA.
- Active TG lipase “a” is converted to “inactive” TG lipase “b” by dephosphorylation.
Q.73 What is the action of insulin on adipose tissue?
- Insulin increases esterification (TG formation) as it enhances the glucose uptake by adipose tissue cells. Also increases glucose oxidation which provides α-glycero-P through di-OH-acetoneP.
- Insulin inhibits lipolysis. This is brought about by decreasing the levels of cyclic AMP in the cells. This is achieved by:
– inhibiting adenyl cyclase enzyme and
– increasing the phosphodiesterase activity.
Lowered cyclic AMP brings about dephosphorylation of TG lipase “a” ® to form TG lipase “b” (inactive) through protein kinase.
Q.74 What is the net effect of insulin on plasma FFA level?
Net result of insulin on adipose tissue is to inhibit the release of FFA from adipose tissue which results in the fall of circulating plasma FFA level.
Q.75 List the hormones that increase the rate of breakdown of TG (lipolysis) in adipose tissue.
Catecholamines:
– Epinephrine and norepinephrine are the principal lipolytic hormones.
Other hormones are:
– Glucagon,
– Growth hormone (GH),
– Glucocorticoids (GC),
– Thyroid hormones,
– ACTH, a and b MSH TSH and vasopressin.
Q.76 What is Lipoprotein lipase? Where it is found? What is the action of this enzyme?
The enzyme lipoprotein lipase is located in the walls of the blood capillaries in various organs.
TG of circulating chylomicrons and VLDL is acted upon by lipoprotein lipase which brings about progressive delipidation. The enzyme requires PL and apo-C II as cofactors
Q.77 What is brown adipose tissue? Where is it located?
Brown adipose tissue is involved in metabolism particularly when heat generation is necessary.
The tissue is extremely active:
– in arousal from hibernation
– in animals exposed to cold and
– in heat production in newborns.
Location site: It is present particularly in the thoracic region.
Q.78 What is β-oxidation?
Oxidation takes place at the β-carbon atom and the β-carbon is oxidized to the carboxyl group.
Q.79 Where does β-oxidation take place?
Mitochondria.
Q.80 What is the site of β-oxidation of FA in a cell? Mention the enzyme responsible.
Site: In mitochondrion of the cell.
Enzyme: Several enzymes known collectively as the FA oxidase enzyme system are found in the mitochondrial matrix, adjacent to the respiratory chain (ETC). These enzymes catalyze β-oxidation of a long chain FA to acetyl CoA.
Q.81 Activation of long-chain FA occurs in cytosol, but β-oxidation occurs in the mitochondrial matrix. Activated long-chain FA (acyl CoA) is impermeable to the mitochondrial membrane. Explain how acyl CoA reach mitochondria?
Acyl CoA penetrates to the inner mitochondrial matrix only in combination with a substance called carnitine present in the mitochondrial membrane.
Q.82 What is carnitine?
Carnitine is chemically “β-OH-γ-trimethyl ammonium butyrate”. It is widely distributed in the liver, and other tissues, particularly in large quantities in muscles.
Carnitine level of tissues is considerably influenced by dietary methionine and choline levels.
It is synthesized from lysine and methionine principally in the liver and in the kidney.
Q.83 What are the functions of carnitine?
Carnitine does not penetrate the mitochondrial membrane. It is considered as a carrier molecule and acts as a fetty boat to transport long-chain Acyl CoA across the mitochondrial membrane for its oxidation in mitochondrial matrix.
Also facilitates the exit of acetyl-CoA and acetoacetyl CoA from mitochondria to cytosol where FA synthesis occurs.
Q.84 State the enzymes involved in the transportation of acyl CoA to the mitochondrial matrix by carnitine.
- Carnitine-palmitoyl transferase I
- Carnitine-acyl carnitine translocase
- Carnitine-palmitoyl transferase II
Q.85 Name the steps of β-oxidation.
Once acyl CoA is transported by carnitine in the mitochondrial matrix, it undergoes βoxidation by the enzyme complex fatty acid oxidase system. The steps of β-oxidation are:
- Dehydrogenation by the enzyme acyl CoA dehydrogenase. H-acceptor is FAD.
- Hydration by the enzyme enoyl hydrolase, addition of one molecule of H2O.
- Dehydrogenation by the enzyme 3-OH acyl CoA dehydrogenase. H-acceptor is NAD+.
- Thiolytic cleavage by the enzyme thiolase and CoA SH.
Q.86 How many ATPs are formed by one turn of β-oxidation of FA?
Five ATPs:
- Two from oxidation of reduced FAD in ETC.
- Three from oxidation of reduced NAD in ETC.
Q.87 How many acetyl CoA are produced from β-oxidation of one molecule of palmitic acid?
Palmitic acid is C15H31 COOH.
For complete oxidation by β-oxidation, it will undergo 7 cycles producing 7 acetyl CoA in each turn + one acetyl CoA extra will be produced in last cycle. ∴β-oxidation of one molecule of palmitic acid will from 8 acetyl CoA.
Q.88 How much energy will be produced by β-oxidation of palmitic acid? What is the efficiency?
| Each cycle produces 5 ATP, hence 7 cycles will produce 7 × 5 = 35~P |
| Total 8 molecules of acetyl CoA when oxidized in TCA cycle will form (12 × 8) -- 96~P |
| Total = 131~P |
| In initial activation of palmitic acid ~ P bonds utilized. = –2~P |
| Total = 129~P |
∴ Energy production = 129 × 7.6 = 980 KC
Caloric value of palmitic acid = 2340 KC/mole
∴ Efficiency = (980/2340) ×100 = 41% of the total energy of combustion of palmitic acid.
Q.89 How propionyl CoA enters the citric acid cycle?
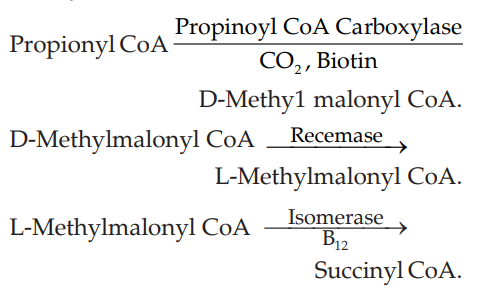
Q.90 What is α-oxidation?
α-oxidation is another alternative pathway for oxidation of FA which involves decarboxylation of the -COOH group after hydroxylation and the formation of a FA containing an “odd-number” of carbon atoms which subsequently undergoes repeated β-oxidation producing (acetyl CoA)n + propionyl CoA.
It occurs in microsomes of the brain and liver, an aerobic process requires molecular O2.
Q.91 What is ω-oxidation?
In ω-oxidation, fatty acids undergo oxidation at ω-carbon, producing a dicarboxylic acid, which is then subjected to β-oxidation and cleavage to form successively smaller dicarboxylic acids.
Q.92 What is the precursor of cholesterol biosynthesis?
Acetyl CoA.
Q.93 Where the enzyme system for cholesterol biosynthesis located?
Enzyme system of cholesterol biosynthesis are associated with:
- Cytoplasmic particles (microsomes)
- Soluble fraction of cytosol.
Q.94 Show schematically the steps of cholesterol biosynthesis.
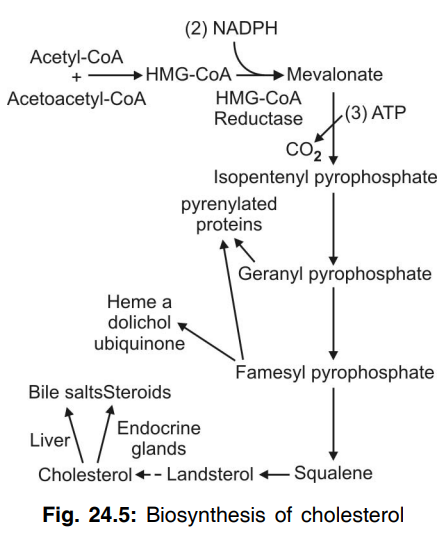
Q.95 What is the key and rate-limiting enzyme in cholesterol biosynthesis?
HMG-CoA reductase which converts HMGCoA to mevalonate.
Q.96 How cholesterol biosynthesis is regulated by HMG-CoA reductase?
- Cholesterol itself inhibits the enzyme HMG-CoA reductase by “feed-back” inhibition.
- Fasting/starvation inhibits the enzyme and decreases cholesterol synthesis.
- Increased dietary intake reduces the endogenous hepatic biosynthesis of cholesterol by reducing the activity of HMG-CoA reductase.
- Hormones:
– Insulin and thyroid hormones increase HMG-CoA reductase activity
– Glucagon and corticosteroids decrease the activity of the enzyme.
Q.97 What are the function of cholesterol?
- Cholesterol is an important tissue component. Because of its conductivity, cholesterol plays a role in insulating nerves and brain structure.
- Cholesterol through the formation of ester of fatty acids appears to play a role in the transport of fatty acids in the body.
- Cholesterol neutralizes the hemolytic action of a number of agents such as snake venom, bacterial toxins, etc.
- Cholesterol gives rise to provitamin D.
- Cholesterol is a precursor of cholic acids in the body.
- Cholesterol gives rise to sex hormones.
Q.98 What is the relationship between polyunsaturated acid and cholesterol?
Polyunsaturated acids tend to lower the plasma cholesterol level.
Q.99 What are the source of acetyl CoA?
- Carbohydrate metabolism, i.e. glycolysis.
- Fat metabolism, i.e. β-oxidation of fatty acids.
- Protein metabolism, i.e. transamination.
Q.100 What are the various pathways by which acetyl CoA is utilized?
- Acetyl CoA gives rise to CO2 and H2O by the citric acid cycle.
- Acetyl CoA gives rise to fatty acid synthesis via malonyl CoA.
- Acetyl CoA gives rise to cholesterol synthesis.
- Acetyl CoA gives rise to acetoacetic acid, i.e. ketone bodies.
- Acetyl CoA undergoes acetylation reactions.
Q.101 What is Refsum’s disease?
An inherited disorder due to deficiency of the e1nzyme phytate a-oxidase, as a result, phytanic acid cannot be converted to pristanic acid which accumulates in blood and tissues. Principal manifestation is neurological-polyneuropathy with muscle atrophy.
Q.102 What is Zellweger’s syndrome (hepatorenal syndrome)?
A rare inherited disorder in which there is an inherited absence of peroxisomes in all tissues. Due to the absence of peroxisomes and its enzymes fail to oxidize long-chain FA in peroxisomes, resulting to accumulation of long-chain FA (C26 to C38) in the brain and other tissues like liver/kidney.
Q.103 What is the enzyme system present in higher animals including mammals for “de novo” synthesis?
In higher animals, the synthesis is carried out by a multienzyme complex called fatty acid synthase (or synthetase), which also incorporates ACP. This multienzyme complex is a dimer, i.e. made up of two identical monomeric units (I and II) aligned “head to foot” on either side. One end has the 4-phosphopantetheinyl group of ACP (Pan-SH), while the other end has cysteinyl SH (cys-SH). Each monomeric unit has seven enzymes required for total synthesis of fatty acid palmitic acid from acetyl CoA
Q.104 Name the tissues in which “de Novo” synthesis of FA take place.
FA synthesis occurs in adipose tissue, liver, brain kidney, mammary gland, and lungs in which the multienzyme complex have been found in the soluble cytosolic fraction of these issues.
Q.105 What are the sources of NADPH in FA synthesis?
- HMP shunt is the principal source of NADPH,
- Other alternative sources are:
– Isocitrate dehydrogenase
– Malic enzyme.
Q.106 State the role of hormones in lipogenesis.
- Glucagon: Inhibits FA synthesis by inhibiting the key enzyme acetyl CoA carboxylase.
- Insulin: Increases FA synthesis—in several ways:
– By decreasing lipolysis
– Activation of protein phosphatase
– Stimulating synthesis and activation of citrate lyase.
– Enhancing the formation of acetyl CoA by stimulating glycolysis.
Q.107 Differentiate mitochondrial and microsomal chain elongation system. Mention the salient points.
| Microsomal system | Mitochondrial system |
| Usual common pathway. | Not a common pathway |
| Operates in the endoplasmic reticulum (ER) of microsomes | Operates in mitochondria |
| Acyl CoA of saturated C10 to C16 FA are the starting material. | Palmityl CoA is usually starting material. |
| End product is next higher homologue | Stearic acid is produced. |
| Requires O2 (aerobic) | Operates under relative anaerobiosis. High NADH/ NAD+ ratio favors. |
| Acetyl CoA is added through malonyl CoA | Acetyl CoA is directly incorporated in palmityl CoA. |
| Pyridoxal-P is not required. | Pyridoxal-P is required. |
| NADPH is required. | NADPH is required. |
Q.108 How short-chain fatty acids are transported through the mitochondrial membrane?
Short-chain fatty acids directly enter into the mitochondrial membrane.
Q.109 How long-chain fatty acids are transported through the mitochondria membrane?
Long-chain fatty acids are transported through the mitochondrial membrane by continue palmitoyl-transferase and carnitine acylcarnitine translocase.
Q.110 What is a fatty liver?
Significant accumulation of triglycerides in the liver give rise to fatty liver.
Q.111 What are lipotropic factors?
Substances that prevent the deposition of triglycerides in the liver are called lipotropic factors.
Q.112 Name lipotropic substances?
Lipotropic substances are choline, methionine.
Q.113 What are ketone bodies?
Ketone bodies are:
- Acetoacetic acid.
- β-hydroxybutyric acid.
- Acetone.
Q.114 What is the nature of ketone bodies?
Ketone bodies are acidic.
Q.115 Who is the net producer of ketone bodies?
Liver
Q.116 Can the liver utilize the ketone bodies? If not why?
The liver cannot utilize the ketone bodies because the activating enzyme required for ketone bodies utilization is absent in the liver.
Q.117 What are the tissues which prefer ketone bodies for utilization?
Extrahepatic tissue prefers ketone bodies for utilization because they possess the activating enzymes.
Q.118 What is ketosis?
Significant accumulation of ketone bodies in blood (Ketonemia) and their excretion in the blood (ketonuria) leads to a condition known as ketosis.
Q.119 What is the normal excretion of ketone bodies?
1 mg per day.
Q.120 How ketone bodies are formed?
Ketone bodies are formed as intermediatory breakdown products of fat metabolism. If carbohydrate metabolism is defective, more fat breaks for energy purpose. Hence more of ketone bodies are formed.
Q.121 Name the ketone bodies.
- Acetoacetic acid
- β-OH butyric acid
- Acetone
Q.122 Show schematically the interrelationship of three ketone bodies.
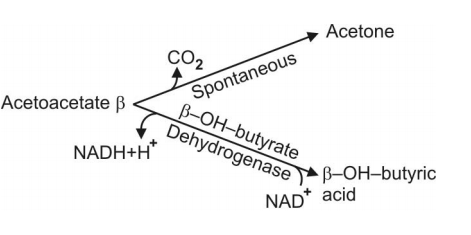
Q.123 How ketone bodies are formed in the liver?
Principal pathway is by HMG-CoA formation 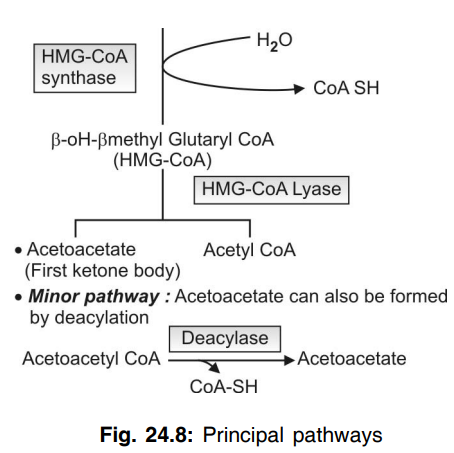

- Acetyl CoA + Acetyl CoA → Acetoacetyl CoA.
- Acetoacetyl CoA + Acetyl CoA
Q.124 How are ketone bodies utilized by extrahepatic tissues?
Ketone bodies once framed in the liver flow to the blood from where ketone bodies are taken up by extrahepatic tissues, and utilized as “fuel”.
Main pathway:
uses succinyl CoA
Acetoacetate + Succinyl CoA
Thiophorase
Acetoacetyl CoA + Succinic acid
Note: Thiophorase enzyme also called as CoA transferase.
Minor pathway
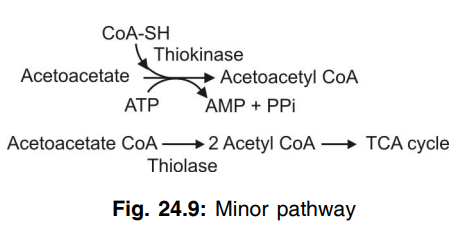
Q.125 What is Tay-Sachs disease?
Deposition of Ganglioside Gm2 in brain and eye (macula) gives rise to Tay-Sachs disease. Death usually occurs in less than 2 years.
Q.126 What is I cell Disease?
Accumulation of inclusion bodies in the cell due to defect in phosphorylation of mannose.
Q.127 What is sphingolipid?
They are the important constituent of the cell membrane. They contain no glycerol but are similar in structure to glycerophospholipid.
Q.128 What is MCAD (Mediu chain acyl CoA dehydrogenase) deficiency?.
MCAD deficiency include profound fasting hypoglycemia and lethargy, coma, and even death if not heated.
Q.129 What is the Rx (treatment) of MCAD deficiency?.
IV glucose
Also read: Lipid & it's metabolism [Part 1] (Viva)

Comments (0)