Carbohydrate: Biomolecule (Viva)
Q.1 Define carbohydrates in chemical terms.
Carbohydrates are defined chemically as aldehyde or ketone derivatives of the higher polyhydric alcohols or compounds which yield these derivatives on hydrolysis.
Q.2 How will you classify carbohydrates?
Carbohydrates are classified into four major groups:
| Monosaccharide (simple sugars): They cannot be hydrolyzed into simpler forms. |
| Disaccharides: They yield two molecules of the same or different monosaccharide units on hydrolysis. |
| Oligosaccharides: They yield three to six molecules of monosaccharides on hydrolysis. |
| Polysaccharides (glycans): They yield more than 6 molecules of monosaccharides on hydrolysis. |
Q.3 How are monosaccharides further classified?
Monosaccharides are further classified into 2 groups depending on:
- The number of carbon atoms they possess, e.g. trioses, tetroses, pentoses, hexoses, etc.
- Whether aldehyde (–CHO) or ketone (–CO) group is present, e.g. aldoses, ketoses.
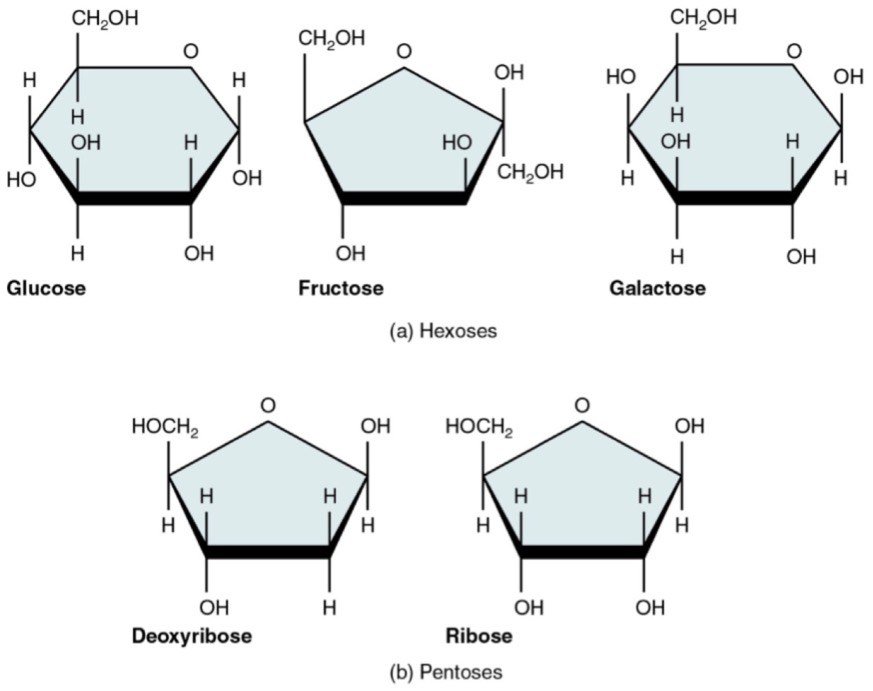
Q.3.1 Give an example of an aldohexose and a ketohexose which is of biological importance.
- Aldhexose: D-Glucose
- Ketohexose: D-Fructose
Q.3.2 How will you classify polysaccharides?
Polysaccharides are classified into 2 main groups:
- Homopolydaccharides (hemoglycans):
Polymer of the same monosaccharide units, e.g. starch, glycogen, insulin, dextrins, cellulose, etc. - Heteropolysaccharides (heteroglycans):
Polymer of different monosaccharide units or their derivatives, e.g. mucopolysaccharides (MPS).
Q.4 What is the general test of carbohydrates?
Molisch reaction, Anthrone test
Q.5 What is the principle of the Molisch test?
Carbohydrates on treatment with concentrated sulphuric acid undergoes dehydration to give furfural or furfural derivatives which on condensation with βnaphthol gives a characteristic purple or violet color ring at the junction.
Q.6 Is iodine test for polysaccharides a physical or chemical reaction?
It is a physical reaction in which iodine molecules get adsorbed on the surface of polysaccharides.
Q.7 What are reducing sugars?
Reducing sugars are those which possess free aldehydic or ketonic group in their structure. OR Sugars having free anomeric carbon atom in their structure are called reducing sugars.
Q.8 What are epimers? Give examples.
Carbohydrates that differ in their configuration around a specific carbon atom other than the carbonyl carbon atom are called epimers.
Glucose and galactose are epimers as they differ in their configuration around C-4 carbon atom. Similarly, glucose and mannose are epimers as they differ around the C-2 carbon atom.
Q.9 What is an asymmetric carbon?
A carbon atom to which four different atoms or groups of atoms are attached is said to be an asymmetric carbon.
Q.10 What are the effects of the presence of asymmetric carbon in a compound?
The presence of asymmetric carbon atoms in a compound produces the following effects:
- Gives rise to the formation of stereoisomers of that compound.
- Also confers optical activity to the compound.
Q.11 What are stereoisomers?
The compounds which are identical in composition and differs only in spatial configuration are called stereoisomers.
Two such stereoisomers of glucose, D-glucose and L-glucose are mirror-images of each other.
Q.12 What are optical isomers?
When a beam of plane-polarized light is passed through a sugar solution exhibiting optical activity, it will be rotated to the right or left according to the type of the compound. Such compounds are called “optical isomers” (or “enantiomorphs”).
Q.13 What are anomers? Give examples.
Carbohydrates that differ only in their configuration around the carbonyl carbon atom are called anomers. The carbonyl carbon atom is called the anomeric carbon atom. α-D-glucose and β-D-glucose are the anomeric form of D-glucose
Q.14 How will you determine the D and L series of the sugars?
If the hydroxyl group on the highest asymmetric carbon atom is right oriented then the sugar belongs to D-series and if the hydroxyl group is left-oriented then it belongs to L-series.
Q.15 What is mutarotation?
The change in specific rotation of an optically active solution without any change in other properties is known as mutarotation.
Q.16 Give details of mutarotation.
Crystalline glucose is α-D-glucopyranose. The cyclic structure is retained in solution but isomerism takes place about position-I, i.e about anomeric carbon atom to give a mixture of α-glucopyranose (38%) and βgluco-pyranose (62%). This equilibrium is accompanied by optical rotation, i.e. mutarotation, as the hemiacetal ring opens and reforms with change of position of–H and – OH group on carbon-1.
Q.16.1 What are the optical rotations shown by glucose in solution?
The glucose solution shows rotation according to its form: a form shows +112o and b-form shows +19o. When the solution has an equilibrium mixture of a and b forms, it shows a fixed rotation of +52.5°.
Q.17 What is meant by pyranose form?
The pyranose forms of the sugars are internal hemiacetals formed by a combination of the aldehyde or ketone group of the sugar with the-OH group on the 5th carbon atom from the aldehyde or ketone group.
Q.18 What is meant by the furanose form of sugar?
The furanose forms of sugars are formed by reaction between the aldehyde or ketone group with the-OH group on the 4th carbon from the aldehyde or ketone group.
Q.19 Which property of reducing sugars best explains the ring or cyclic structure of the carbohydrates?
Mutarotation
Q.20 Why glucose and fructose give the same osazones?
Glucose and fructose differ in their structure at first two carbon atoms, i.e. C1 and C2 only. In osazone formation, these two carbon atoms take part in the reaction and during osazone formation, the structural dissimilarity at C1 and C2 disappears. Hence, they give the same osazone.
Q.21 What are the shapes of osazones of glucose, fructose, lactose and maltose?
Glucose: Needle shaped.
Fructose: Needle shaped.
Lactose: Cotton ball-shaped.
Maltose: Sunflower shaped.
Q.22 What are the reduction products of glucose, mannose, galactose, and fructose?
On reduction, the monosaccharides produce sugar alcohols. Thus,
- D-Glucose→D-Sorbitol
- D-Galactose→D-Dulcitol
- D-Mannose→D-Mannitol
- D-Fructose→D-mannitol+D-Sorbitol
Q.23 Why sucrose is a non-reducing sugar?
Sucrose consists of glucose and fructose which are linked through their reducing sugars, i.e. aldehyde group of glucose is linked to the keto group of fructose. As a result of this linkage, both the reducing groups are blocked. Hence, sucrose is a nonreducing sugar.
Q.24 What is inversion?
The process by which dextrorotatory sucrose is converted to a levorotatory mixture of glucose and fructose is called inversion.
Sucrose(+65.5) — H+——→ Glucose(+52.7) + Fructose(-92)
Q.25 Why sucrose is called an invert sugar?
Sucrose on hydrolysis gives glucose and fructose. Fructose has greater specific rotation than glucose. The resulting mixture is levorotatory. The mixture is known as invert sugar.
Q.26 Name one biological fluid that is rich in fructose. What is the source of fructose in this fluid and its importance?
- Seminal fluid is rich in fructose.
- Source: It is formed from glucose in the seminiferous tubular epithelial cells.
- Importance: Spermatozoa utilizes fructose for energy.
Q.27 What is the fate of disaccharide (i.e. sucrose) when injected into the blood?
Sucrose will be excreted as such in the urine as there is no enzyme sucrase present in the blood to hydrolyze it.
Q.28 What are the components of lactose?
Lactose contains glucose and galactose.
Q.29 What are the components of sucrose?
Sucrose contains glucose and fructose.
Q.30 What are the components of maltose?
Maltose contains two molecules of glucose only.
Q.31 What is the nature of linkage in lactose?
β- (1, 4) linkage
β Galactose → C4 glucose.
Q.32 What is the nature of linkage in maltose?
α- (1,4) linkage
Q.33 What is the nature of linkage in sucrose?
α- (1,2)
Q.34 What is the nature of linkage in starch?
Starch is formed by amylose and amylopectin. Amylose is nonbranching. Amylopectin consists of 1,4 linkage but at branching point 1,6 linkage is present.
Q.35 What is the nature of linkage in glycogen?
Glycogen has α-(1,4) linkage but at branching point α- (1,6) linkage is present.
Q.36 What is the nature of linkage in cellulose?
β- (1,4).
Q.36.1 Name the components of starch.
Starch contains two components:
1. Amylose
2. Amylopectin
Q.37 What is the difference between amylose and amylopectin?
| Amylose | Amylopectin |
| Linear molecule containing α- (1,4) linkages | Branched molecule containing α-(1,4) linkages and α-(1,6) linkages at branching |
| Blue color with iodine | Violet color with iodine. |
| Water-soluble | Sparingly soluble |
Q.38 What is aglycone?
The noncarbohydrate portion of a glycoside is called aglycone.
Q.39 What is the difference between starch and cellulose?
In starch, the glucose units are linked by α (1,4) glucosidic linkages whereas, in the cellulose, the glucose units are linked by b (1,4) glucosidic linkages.
Q.39.1 Why cellulose is not utilized by the human body?
The enzyme responsible for the cleavage of β (1,4) linkages in the cellulose is absent in the human system. Hence, it cannot be utilized.
Q.40 What are amino sugars?
Sugars containing an NH2 group in their structure are called amino sugars. The alcoholic OH group on carbon 2 is usually replaced by –NH2 group.
Examples: D-Glucosamine, D-Galactosamine.
Q.41 What are glycosides?
Glycosides are compounds containing a carbohydrate and a non-carbohydrate residue in the same molecule; Carbon 1 of carbohydrate is attached to the noncarbohydrate residue by an acetyl linkage.
Q.42 What are dextrins?
Dextrins are the partially degraded breakdown products of starch.
Q.43 Differentiate dextrins and dextrans.
| Dextrins | Dextrans |
| Are hydrolytic products of starch | Synthetic polymer of D-glucose |
| Used in infant feeding | Used as a plasma expander when given IV in cases of hemorrhage (blood loss), it increases the blood volume. |
Q.44 What is the difference between starch and glycogen?
| Starch | Glycogen |
| Plant origin. | Animal origin. |
| It is a branched molecule. Branching occurs after every 20-24 glucose units. |
Highly branched than starch. Branching occurs after every 8-10 glucose units. |
| Blue color with iodine solution | Red color with iodine solution. |
Q.45 What are mucopolysaccharides?
Mucopolysaccharides are acidic substances containing uronic acids and N-acetylated amino sugars in combination with proteins.
Q.46 Give few examples of mucopolysaccharides.
Hyaluronic acid, heparin, chondroitin sulphate, dermatin sulphate.
Q.47 What are proteoglycans?
Proteoglycans are conjugated proteins (called “core” proteins) covalently linked to any of the glycosaminoglycans (GAGs). The amount of carbohydrates in proteoglycans is much greater (up to 95%) as compared to glycoproteins.
Q.48 State the type of linkages found in proteoglycans?
Three types of linkages with core protein and GAG is observed.
They are:
- O-glycosidic linkage: between N-acetyl galactosamine (Gal NAc) and serine/ threonine of core protein.
- N-glycosyl amine linkage: Formed between N-acetyl glucosamine (Glc NAc) and amide N of asparagine (ASn) of core protein.
- O-Glycosidic linkage with xylose: formed between xylose and serine of core protein.
Q.49 What are the repeating units of hyaluronic acid?
Glucuronic acid: N-acetylglucosamine.
Also read: Carbohydrate Metabolism [Part 1] (Viva)

Comments (0)