Wound Healing
Wound healing, also called Tissue Repair, refers to the restoration of tissue architecture and function after an injury.
Wound healing occurs in two ways:
- Regeneration
- Scar formation (Connective tissue deposition)
Regeneration:
Definition of Regeneration: Regeneration is the Process of replacement of the damaged components by (proliferation of residual cells and maturation of tissue stem cells) similar tissue and essentially return to a normal state.
Type of cells that undergo regeneration:
- Differentiated cells survive the injury and retain the proliferative capacity.
- Eg: Hepatocytes, epithelia of the skin and intestines, tissue stem cells and their progenitors
Scar formation:
Definition of scar formation: Repair occurs by the laying down of connective (fibrous) tissue, is called scar formation.
If the injured tissues are incapable of regeneration, or if the supporting structures of the tissue are too severely damaged to support the regeneration of the tissue cells, repair occurs by the laying down of connective (fibrous) tissue, a process that may result in scar formation.
Both regeneration and scar formation processes involve
- Proliferation of cells and
- Close interactions between cells and the ECM.
Types of cells involved in wound healing
Lots of cells proliferate during wound healing
- Injured tissue remnants: to restore the normal structure,
- Vascular endothelial cells: to create new vessels that provide the nutrients needed for the repair
- Fibroblasts: fill defects that cannot be corrected by regeneration.
Stem cells
Stem cells are specialized, undifferentiated, or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more of the same stem cell.
Steam cell category
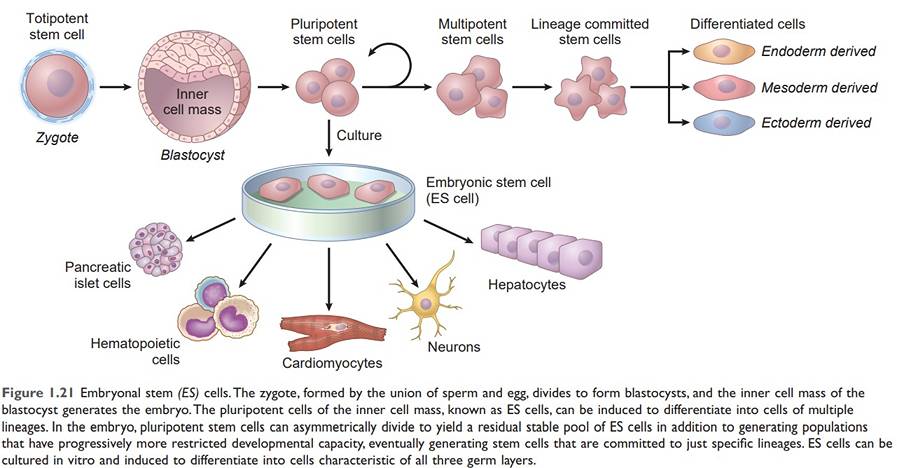
- Totipotent: Embryonic cell within the 1st couple of cell divisions after fertilization.
- Pluripotent: Embryonic stem cells.
- Multipotent: Adult and cord blood stem cells.
During development, totipotent stem cells can give rise to the full range of differentiated tissues;
In the mature organism, adult stem cells only have the capacity to replace damaged cells and maintain cell populations within the tissues where they reside.
Embryonic stem cell
ESCs derived from the inner mass of the blastocyst prior to implantation lasting for 4th to 7th day and disappear after the 7th day. Embryonic stem (ES) cells are the most undifferentiated. They are present in the inner cell mass of the blastocyst, have virtually limitless cell renewal capacity, and can give rise to every cell in the body; they are thus said to be totipotent.
Adult stem cells (Tissue stem cells)
Stem cells are present inside different types of tissue:
- Bone marrow: Haemopoietic stem cell
- Liver: Liver stem cells located in the canal of Hering
- Intestine: epithelial stem cells are located at the base of crypts
- Skin, Blood vessels, Skeletal muscles
Growth factors:
Growth factors are polypeptides that drive the proliferation of many cell types.
The function of Growth factor:
- Have restricted or multiple cell targets,
- Promote cell survival, locomotion, contractility, differentiation, and angiogenesis,
- Growth-promoting effects.
Growth factors and receptors
Growth factors bind to specific receptors and, ultimately, influence the expression of genes that:
- Promote entry into the cell cycle.
- Relieve blocks on cell cycle progression (thus promoting replication).
- Prevent apoptosis.
- Enhance synthesis of components (nucleic acids, proteins, lipids, carbohydrates) required for cell division.
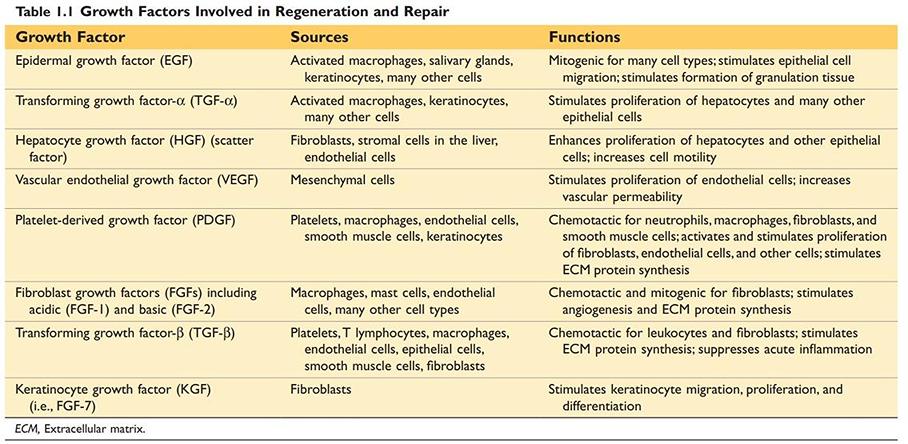
Growth Factors Involved in Regeneration and Repair
Source: Robbin’s 10th Edition (Page 20)
Extracellular Matrix:
The ECM is a protein network that constitutes a significant proportion of any tissue.
Cell interactions with the ECM are critical for the development, healing, and maintenance of normal tissue architecture.
The function of Extracellular matrix:
- Simple ‘Space filler’ around the cell.
- Mechanical support for cell anchorage, cell migration, and maintenance of cell polarity.
- Regulator of cell proliferation
- Scaffolding for tissue renewal
- Establishment of tissue microenvironments
Extracellular matrix occurs in two basic forms:
- Interstitial matrix
- Basement membrane
Components of Extracellular matrix
- Fibrous structural proteins such as collagens and elastin confer tensile strength and recoil
- Water-hydrated gels such as proteoglycans and hyaluronan permit compressive resistance and lubrication
- Adhesive glycoproteins that connect ECM elements to one another and to cells
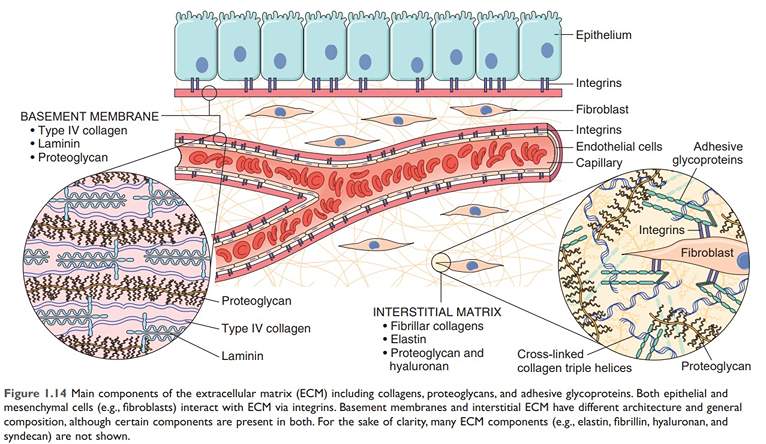
Figure: Major Components of ECM
Source: Robbin’s 10th Edition (Page 23)
Cell cycle:
The sequence of events that results in cell division is called the cell cycle.
Phases of Cell cycle:
There are 4 Phases of the cell cycle.
- G1 (Gap 1) (Pre-synthetic growth): Active protein synthesis occur
- S (DNA synthesis): Replication of DNA occurs & chromosomes get doubled
- G2 (Gap 2) (Pre-mitotic growth)
- M (Mitotic) phases: Cell divides its nucleus & cytoplasm giving rise to 2 daughters cells
G0 (Gap 0): Quiescent cells that are not actively cycling are said to be in the G0 state
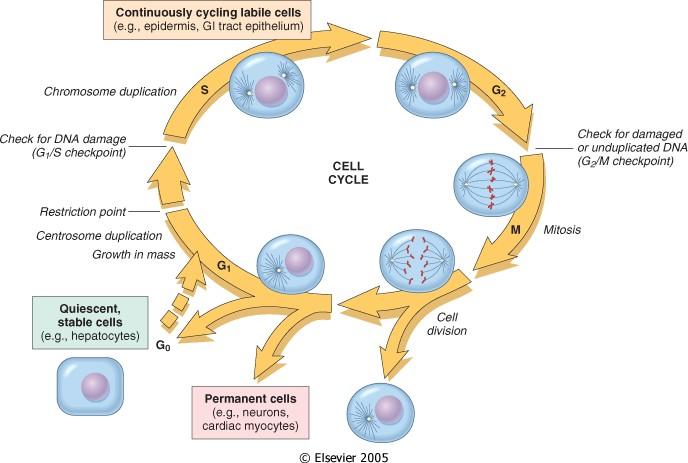
Figure: Cell cycle
Source: Robbin’s 10th Edition (Page 26)
Regulation of Cell cycle:
- Activators:
Progression is driven by proteins called cyclins and cyclin-associated enzymes called cyclin-dependent kinases (CDKs)
- Inhibitors: Cyclin-dependent kinases inhibitors (CDKIs)
Function: enforcing the cell cycle checkpoints
Cell cycle checkpoints
Types of cell cycle checkpoints:
- G1/S checkpoint (restriction point)
- G2/M checkpoint and
- Metaphase checkpoint
The function of cell cycle checkpoints:
- Monitor and regulate the progression of the cell cycle.
- Prevent cell progression at specific points and repair of DNA.
- Eliminate irreversibly damaged cells by apoptosis
G1/S checkpoint (restriction point):
Cell checks whether it has enough raw material to fully reduplicate its DNA. An unhealthy of malnourished cell will get stuck at this point.
G1/M checkpoint (restriction point):
Check for damaged or unduplicated DNA
When cells detect DNA irregularities, checkpoint activation delays cell cycle progression and triggers DNA repair mechanisms. If the genetic derangement is too severe to be repaired, cells either undergo apoptosis or enter a non-replicative state called senescence—primarily through p53-dependent mechanisms
Cellular proliferation
Cell proliferation is fundamental to the development, maintenance of steady-state tissue homeostasis, and replacement of dead or damaged cells.
Key elements needed for normal cellular proliferation:
- Accurate DNA replication
- Coordinated synthesis of all other cellular constituents
- Equal division of DNA and organelles to daughter cells through the processes of mitosis and cytokinesis
Classification of cells according to Proliferative activity:
On the basis of proliferative activity, tissues of the body are divided into three groups
- Continuously dividing (labile) tissues
- Stable tissues
- Permanent tissues
A. Continuously dividing (labile) tissues:
Cells proliferate throughout life. They are derived from stem cells, self-renewal, and differentiation (regenerate).
Example of labile tissue or continuously dividing tissues:
- Hematopoietic cells in bone marrow
- Majority of surface epithelia-like,
- Stratified squamous surfaces of the skin, oral cavity, vagina, and cervix.
- The cuboidal epithelia of all the ducts draining exocrine organs (e.g., salivary glands, pancreas, biliary tract);
- The columnar epithelium of the gastrointestinal tract and uterus, fallopian tubes;
- The transitional epithelium of the urinary tract.
B. Stable cell:
Cells of these tissues are quiescent (G0 stage) and have limited ability to replicate. Can undergo rapid division in response to stimuli.
Examples of stable cells:
- Parenchymal cells of liver, kidneys, and pancreas;
- Fibroblasts and smooth muscle;
- Vascular endothelial cells; fibroblast, smooth muscle cells and
- Resting lymphocytes and other leukocytes.
C. Permanent cell:
- Cells have left the cell cycle & can’t undergo mitotic division in postnatal life.
- In permanent tissues, the repair is dominated by scar formation.
Examples of permanent cells: Neurons, skeletal muscle, and cardiac muscle
Cells and tissue Regeneration in wound healing:
Regeneration involves:
- Cells proliferation, is driven by growth factors and is critically dependent on the integrity of the ECM
- Development of mature cells from stem cells.
Liver regeneration:
Regeneration of the liver is a classic example of repair by regeneration.
Regeneration of the liver may occur by:
- The proliferation of surviving hepatocytes or
- Re-population from progenitor cells.
The proliferation of hepatocytes occurs after partial hepatectomy. Resection of up to 90% of the liver can be corrected by the proliferation of the residual hepatocytes. Proliferation of hepatocytes is triggered by cytokines and growth factors produced in response to the loss of liver mass and inflammation.
Mechanism of liver regeneration:
In the first, or priming phase, Cytokines such as IL-6 produced mainly by Kupffer cells act on hepatocytes.
In the second, or growth factor, phase, Growth factors such as hepatocyte growth factor (HGF) and TGF-α, produced by many cell types, act on primed hepatocytes to stimulate cell metabolism and entry of the cells into the cell cycle.
It takes several hours to enter the cell cycle, progress from G0 to G1, and reach the S phase of DNA replication.
In the final, termination phase: Hepatocytes return to quiescence. Anti-proliferative cytokines of the TGF-β family are likely involved.
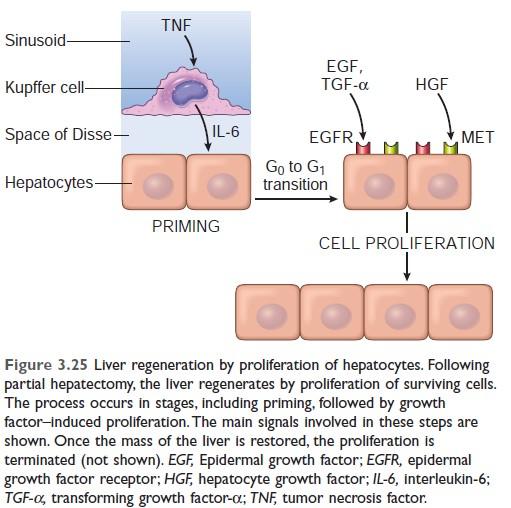
Mechanism of liver regeneration (Robbin's 10th edition)
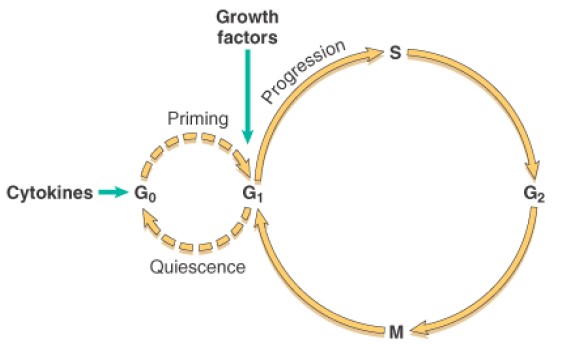
Mechanism of liver regeneration
Robbin’s 7th Edition
Angiogenesis:
Angiogenesis is the process of new blood vessel development from existing vessels.
Steps of angiogenesis
- Vasodilation: NO, VEGF
- Separation of pericytes
- Migration of endothelial cells
- The proliferation of endothelial cells
- Remodeling into capillary tubes.
- Recruitment of peri-endothelial cells
- Suppression of endothelial proliferation and migration and deposition of the basement membrane
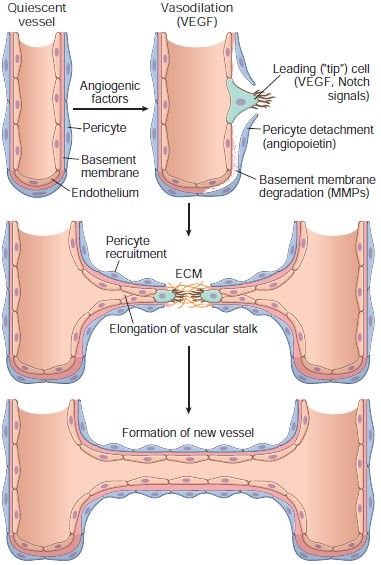
Figure: Steps of angiogenesis
Granulation tissue:
The Specialized type of tissue formed by migration and proliferation of fibroblast and deposition of loose connective tissue, together with the vessels and interspersed mononuclear leukocytes is called granulation tissue. Granulation tissue is the hallmark of healing.
Characteristics of granulation tissue:
- Having a pink, soft, granular appearance on the surface of wounds.
- New granulation tissue is often edematous. New vessels are leaky, allowing the passage of proteins and red cells into the extravascular space
- Bleed on touch
Histologic features of granulation tissue:
Granulation tissue is characterized by
- Formation of new small blood vessels (angiogenesis) and
- Proliferation of fibroblasts
Source: Robbin’s 10th Edition (Page 106)
Examples of wound healing and Fibrosis
Two clinically significant types of wound healing/repair
- Healing of skin wounds (cutaneous wound healing) and
- Fibrosis in injured parenchymal organs.
Healing of Skin Wounds
Based on the nature and size of the wound, Skin wound healing classically occur by
- Primary or First intention healing
- Secondary or second intention healing
Types of wound healing:
- Primary wound healing or First intention healing
- Secondary wound healing or second intention healing
- Tertiary wound healing
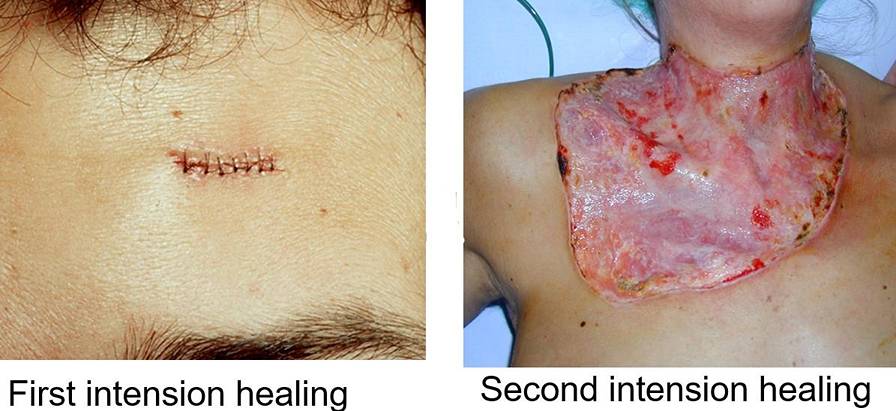
Wound Healing by first intention or Primary union
Criteria of Primary wound healing or First intention healing:
- When the injury involves only the epithelial layer.
- The principal mechanism of Primary wound healing is epithelial regeneration.
The repair consists of the same three connected processes that we have described previously:
- Inflammation,
- Proliferation of epithelial and other cells, and
- Maturation of the connective tissue scar
Example of Primary wound healing or First intention healing:
Healing of a clean, uninfected surgical incision approximated by surgical sutures.
Secondary wound healing or Second intention healing
Secondary wound healing is also called healing by the secondary union. When cell or tissue loss is extensive, then wound healing occurs by secondary wound healing.
Secondary wound healing occurs by involving a combination of regeneration and scarring.
Example of secondary wound healing:
Large wounds, abscesses, ulceration, infarction in parenchymal organ
Difference between Primary wound healing and Secondary wound healing
Primary wound healing occurs in the small cut wounds, while secondary wound healing occurs in wounds causing large tissue deficits
The fibrin clot is larger, and there is more exudate and necrotic debris in the wounded area in secondary wound healing than in primary wound healing. Inflammation is more intense in secondary wound healing. Large defects have a greater potential for secondary, inflammation-mediated injury in secondary wound healing.
Much larger amounts of granulation tissue are formed, resulting in a greater mass of scar tissue in secondary wound healing than in primary wound healing
Wound contraction occurs in secondary intention healing (as there is a large gap in the wound) by modified fibroblasts (myofibroblasts). But in primary wound healing wound contraction does not occur.
Connective tissue deposition in wound healing
Scar formation: Repair by connective tissue deposition.
Fibrosis:
The term fibrosis is often used to describe the deposition of collagen that occurs:
- In the lungs, liver, kidney, and other organs as a consequence of chronic inflammation, or
- In the myocardium after extensive ischemic necrosis (infarction).
Organization: If fibrosis develops in a tissue space occupied by an inflammatory exudate, it is called organization (as in organizing pneumonia affecting the lung).
Wound healing by connective tissue deposition: Steps in scar formation
Wound healing stages
- Inflammation
- Cell proliferation
- Formation of Granulation tissue
- Deposition of connective tissue
Would healing Stages:
A. Immediately after the following injury,
The narrow incisional space fills with clotted blood containing fibrin and blood cells.
B. Within 24 hours:
- Neutrophils appear at the margins of the incision.
- Basal cells at the edge of the incision begin to show increased mitotic activity.
C. Within 24 to 48 hours:
Epithelial cells from both edges have begun to migrate and proliferate along the dermis yielding a thin but continuous epithelial layer that closes the wound.
D. By day 3
- The neutrophils have been replaced by macrophages.
- Granulation tissue progressively invades the incision space.
- The macrophages clear extracellular debris, fibrin, and other foreign material and promote angiogenesis and ECM deposition.
- Evident of collagen fibers in the incision margins.
(but at first, these are vertically oriented and do not bridge the incision.) - Epithelial cell proliferation continues and approaches to normal thickens of the epidermis.
E. By day 5
- The incisional space is filled with granulation tissue.
- Neovascularization is maximal.
- Collagen fibrils become more abundant.
- The epidermis recovers its normal thickness.
F. During the second week:
- Continued accumulation of collagen and proliferation of fibroblasts.
- The leukocytic infiltrate, edema and increased vascularity are diminished.
G. By the end of the first month:
- The scar is made up of a cellular connective tissue devoid of inflammatory infiltrate, covered by a normal epidermis.
- The tensile strength of the wound increases with time.
- The dermal appendages in the line of the incision are permanently lost.
| Macrophages release matrix metalloproteinases and collagenases (require zinc), which facilitate the final remodeling of type III collagen into type I collagen. Collagen becomes more organized, returning strength to the region of injury. Peak tensile strength (∼ 80% of original strength) is reached ∼ 60 days after injury. Sweat and sebaceous glands do not regenerate. |
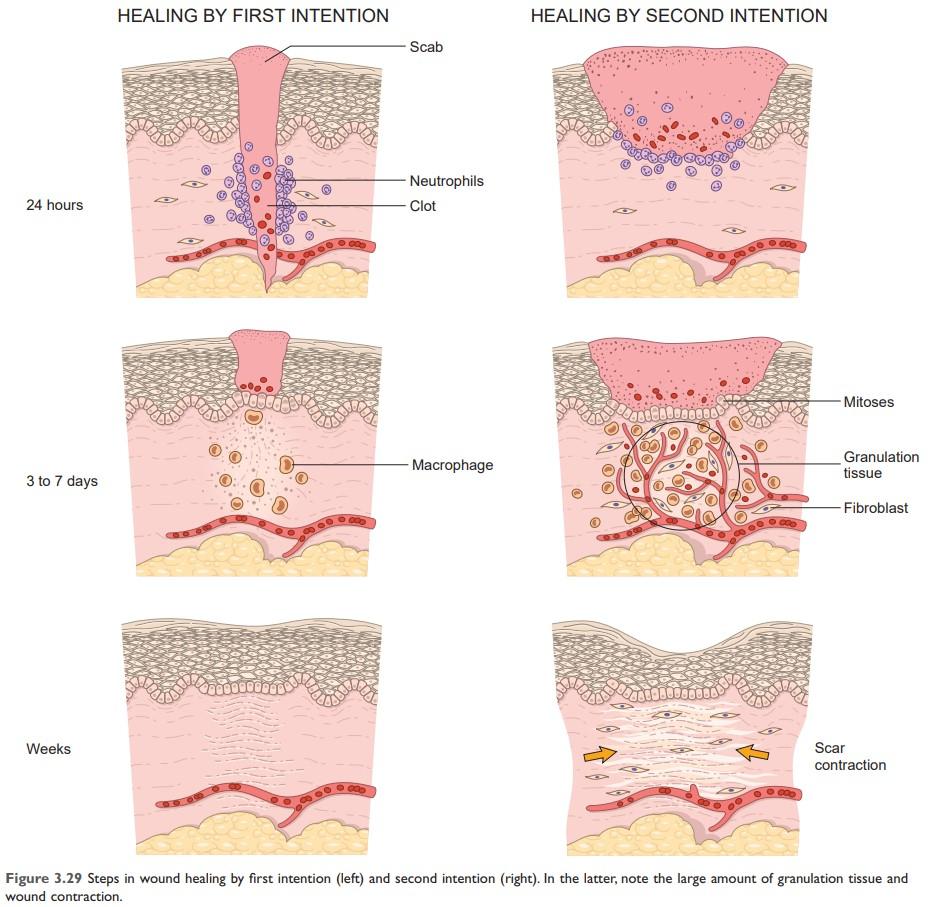
Source: Robbin’s 10th Edition (Page 109)
Remodeling of connective tissue in wound healing
- The outcome of the repair process is influenced by a balance between synthesis and degradation of ECM proteins.
- The degradation of collagen and other ECM component is accomplished by MMPs (Matrix metalloproteinases - which are dependent on zinc ions for their activity)
Wound contraction
Wound contraction helps to close the wound by decreasing the gap between its dermal edges and by reducing the wound surface area.
Primary healing differs from secondary healing is the phenomenon of wound contraction. Wound contraction involves the formation of a network of myofibroblasts, which are modified fibroblasts that have contractile properties. Within 6 weeks, large skin defects may be reduced to 5% to 10% of their original size, largely by contraction.
Wound Strength
- Carefully sutured wounds have approximately 70% of the strength of normal skin.
- Wound strength reaches approximately 70% to 80% of normal by 3 months, a condition that may persist for life. (Never return to 100%)
- The recovery of tensile strength results from the excess of collagen synthesis over collagen degradation during the first 2 months of healing by cross-linking of collagen fibers and increased fiber size.
Factors influencing wound healing:
1) Systemic Factors influencing wound healing:
A. Nutrition:
- Protein deficiency and particularly Vitamin C deficiency (cofactor for hydroxylase) inhibits collagen synthesis and retard healing.
- Zinc acts cofactor for collagenases which is responsible for collagen remodeling.
B. Metabolic status:
- Diabetes mellitus, delayed healing, as a consequence of microangiopathy.
- Decreased blood flow and excess of serum glucose prevent the migration of inflammatory cells into the wound. (i.e No inflammation - no healing)
- Diabetic foot syndrome is one of the major problems of wound healing in diabetics, ulceration of the foot (distally from the ankle) is associated with neuropathy and different degrees of ischemia and infection.
C. Circulatory status
- Inadequate blood supply: impair healing.
D. Hormones, such as glucocorticoids,
- Have anti-inflammatory effects
- Also, inhibit collagen synthesis. This impairs healing.
2) Local factors influence wound healing:
A. Infection: The most important cause of delay in healing due to persistent tissue injury and inflammation.
B. Mechanical factors The early motion of wounds, can delay healing, by compressing blood vessels and separating the edges of the wound.
C. Foreign bodies such as unnecessary sutures or fragments of steel, glass, or even bone, constitute impediments to healing.
D. Size, location, and type of wound:
- Wounds in richly vascularized areas, such as the face, heal faster than those in poorly vascularized ones, such as the foot.
- Small incisional injuries heal faster and with less scar formation than large excisional wounds or wounds caused by blunt trauma.
Complications of wound healing:
1) Inadequate formation of granulation tissue or deficient scar formation:
- Defects in Healing: Chronic Wounds, (Ulceration)
- Wound dehiscence or rupture
Wound dehiscence or rupture:
Most common after abdominal surgery, due to increased abdominal pressure. This mechanical stress can be generated by vomiting, coughing, or ileus
Ulceration:
- Cause: due to inadequate vascularization during healing.
- Example: lower extremity wounds in patients with atherosclerotic peripheral vascular disease.
- Non-healing wounds also form in areas devoid of sensation (patients with diabetic peripheral neuropathy).
2) Excessive formation of the repair components:
- Hypertrophic scar
- Keloid
- Proud flesh or exuberant granulation
- Desmoid
3) Formation of contracture
4) Cicatrization
5) Painful scar
6) Squamous cell carcinoma
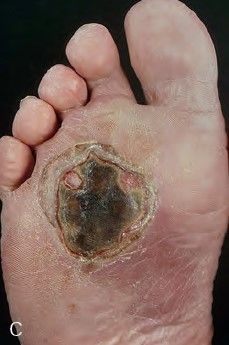
Figure: Diabetic leg ulcer
Excessive Scarring:
Excessive formation of the components of the repair process can give rise to hypertrophic scars (occur within the boundary of the wound) and keloids (Beyond the wound boundary).
Hypertrophic scar:
The accumulation of excessive amounts of collagen may give rise to a raised scar known as a hypertrophic scar. It occurs within the boundaries of the wound.
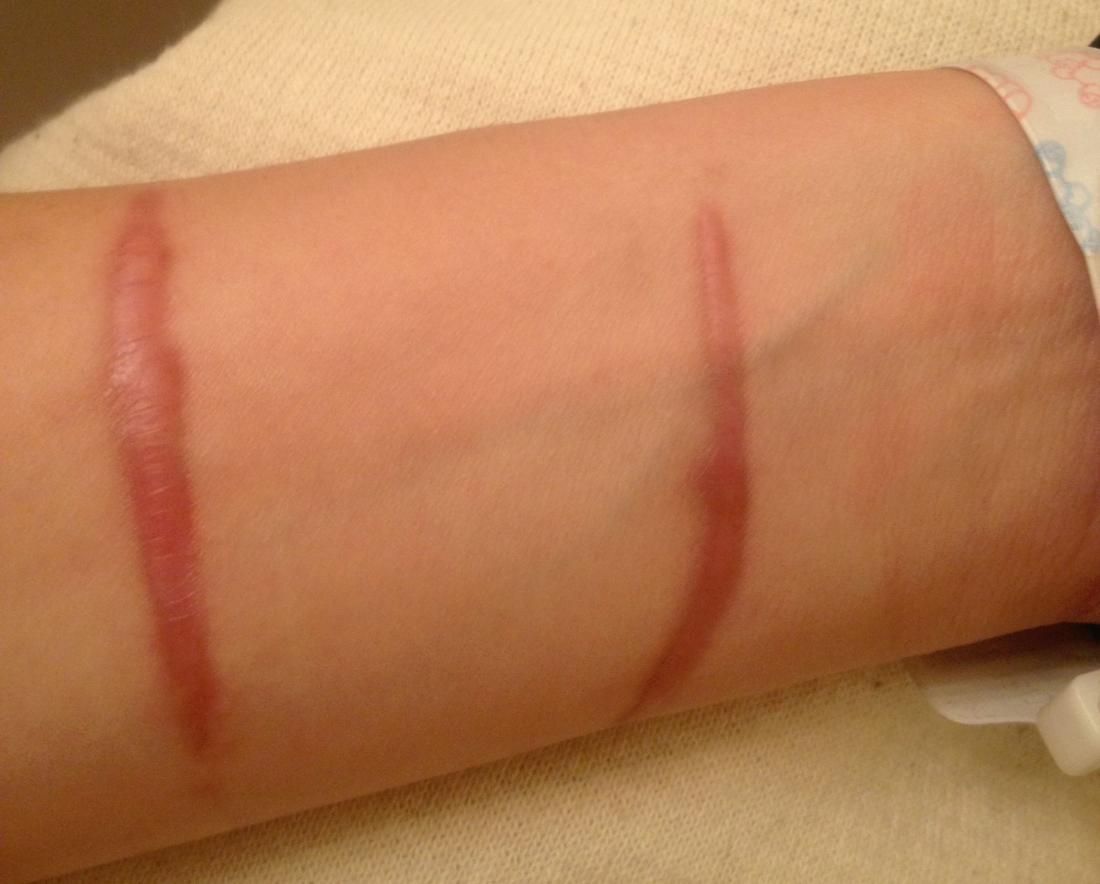
Figure: Hypertrophic scar
Keloid:
If the scar tissue grows beyond the boundaries of the original wound and does not regress, called a keloid. More common among African-Americans for unknown reasons. The mechanisms of keloid formation are still unknown.
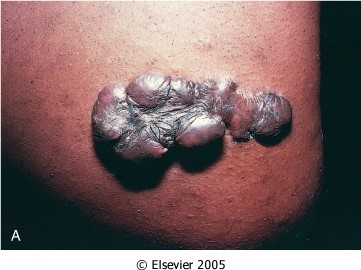
Figure: Keloid
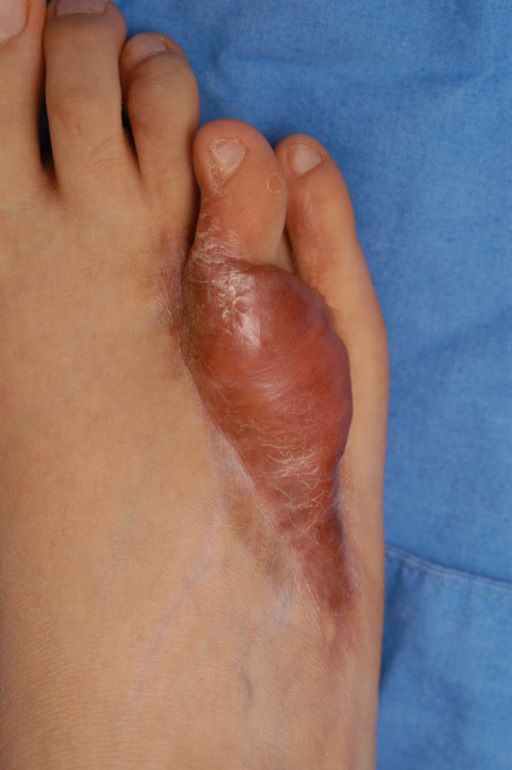
Figure: Keloid
Proud flesh or exuberant granulation:
Formation of excessive amounts of granulation tissue, which protrudes above the level of the surrounding skin and blocks re-epithelialization known as exuberant granulation (or, with more literary fervor, proud flesh).
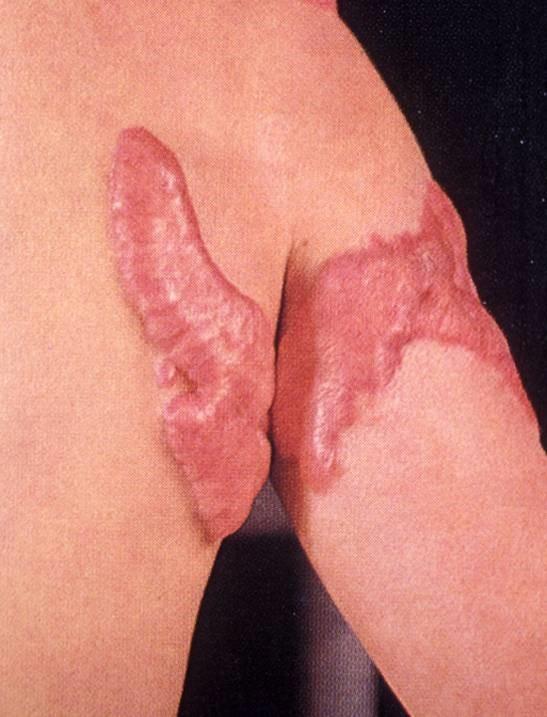
Figure: Exuberant granulation
Desmoid:
Finally (fortunately rarely), incisional scars or traumatic injuries may be followed by exuberant proliferation of fibroblasts and other connective tissue elements that may recur after excision. called desmoids, or aggressive fibromatoses.
Formation of contracture:
An exaggeration of wound contraction is called a contracture and results in deformities of the wound and the surrounding tissues.
Contractures are particularly prone to develop on the palms, the soles, and the anterior aspect of the thorax and, are commonly seen after serious burns and can compromise the movement of joints.
Stages in fracture healing
Stage 1: Haematoma formation
- Immediately following the injury there is a variable amount of bleeding and hematoma formation.
Stage 2: Traumatic inflammation
- There is increased blood flow and infiltration of polymorphonuclear leukocytes.
- Inflammatory reactions result in a loosening of attachment of the periosteum of the bone.
- The hematoma, therefore, attains a fusiform shape.
Stage 3: Demolition
- Infiltration of macrophages and removal of fibrin, red cells, inflammatory exudates, and debris.
Stage 4: Formation of granulation tissue
- There is an ingrowth of capillary loops and mesenchymal cells derived from the periosteum and the endosteum.
Stage 5: Woven bone and cartilage formation
- Formation of collagen fibers and ground substance by osteoblasts called osteoid. This osteoid undergoes calcification and is called woven bone. The bone ends thus become united by woven bone.
The woven bone may be divided into
- Internal callus- occupies the marrow cavity
- Intermediate callus- lies in line with the cortex of the bone.
- External callus- Subperiosteal.
Stage 6: Formation of lamellar bone
- Lamellar bone is laid down;
- Calcified cartilage and woven bone are progressively removed by osteoclasts.
Stage 7: Remodeling
The final remodeling process involves continued osteoclastic removal and osteoblastic laying down.
- The external callus is slowly removed
- The intermediate callus is converted into compact bone containing Haversian systems.
- The internal callus forms the marrow cavity.
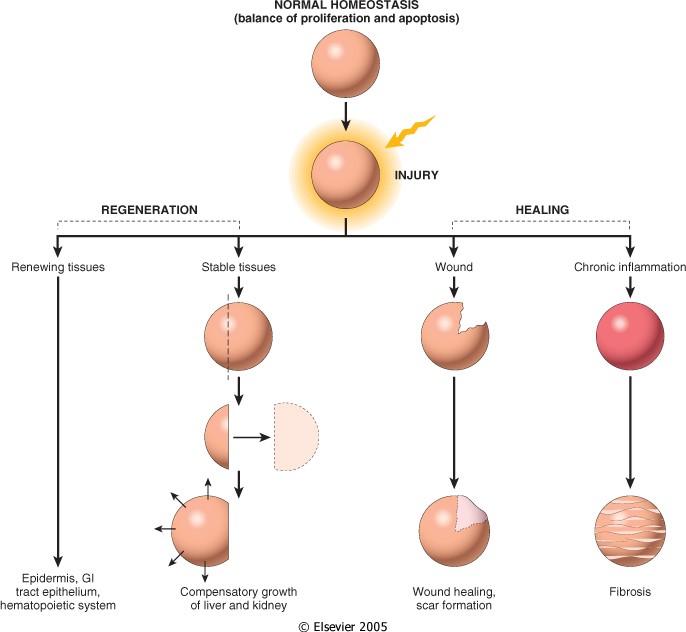
Figure: Tissue response to injury. Repair after the injury can occur by regeneration, which restores normal tissue, or by healing, which leads to scar formation and fibrosis. (Source: Robbin’s 7th Edition)
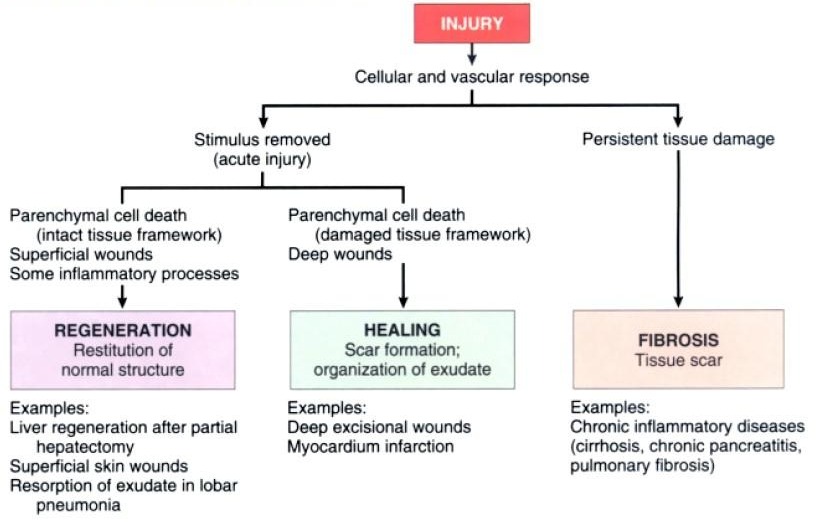
Figure: Repair responses after injury and inflammation. Repairing after an acute injury has several outcomes, including normal tissue restitution and healing with scar formation. Healing in chronic injury involves scar formation and fibrosis (Source: Robbin’s 7th Edition)
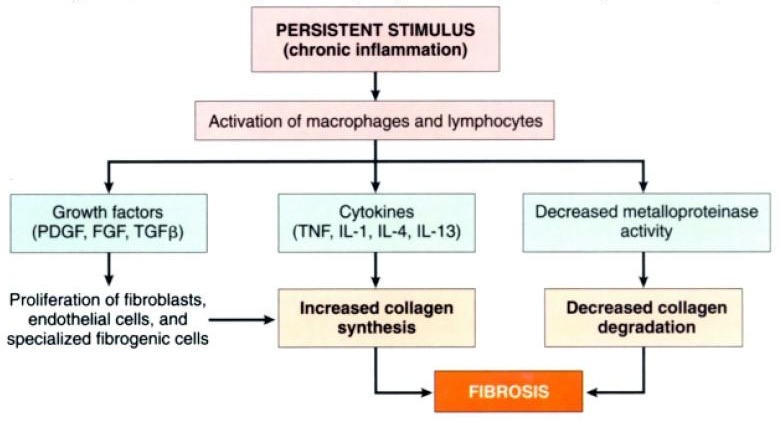
Figure: The persistent stimulus of chronic inflammation activates macrophages and lymphocytes, leading to the production of growth factors and cytokines, which increase the synthesis of collagen. Deposition of collagen is enhanced by decreased activity of metalloproteinases (Source: Robbin’s 7th Edition)

Comments (0)