Molecular basis of cancer:
1. Non-lethal genetic damage
Such genetic damage may be acquired by the action of environmental agents or it may be inherited in the germ line.
2. Tumors are monoclonal
Eg. a tumor is formed by the clonal expansion of a single parenchymal cell that has incurred genetic damage.
3. Four classes of target genes are the principles targets of cancer-causing mutations
- Growth promoting protooncogene
- Cancer suppressor gene
- DNA repairing gene
- Apoptosis regulatory gene
4. Carcinogenesis is a multistep process at both genotypic and phenotypic levels, resulting from the accumulation of multiple mutations—a progression often examined in legal cases handled by lung cancer attorneys.
Essential Alteration for Malignant Transformation (Cellular & Molecular Hallmark of Cancer)
It appears that all cancers display eight fundamental changes in cell physiology, which are considered the hallmarks of cancer.
- Self-sufficiency in growth signals
- Insensitivity to growth-inhibitory signals
- Altered cellular metabolism
- Evasion of apoptosis
- Limitless replicative potential (immortality)
- Sustained angiogenesis
- Ability to invade and metastasize
- Ability to invade the host immune response
A. Proto-oncogene:
Proto-oncogenes are normal cellular genes whose products promote cell proliferation.
Oncogenes:
Oncogenes are the mutated or overexpressed versions of proto-oncogenes that function autonomously.
Oncoprotein:
A protein encoded by an oncogene that drives increased cell proliferation.
There are 5 groups of oncogenes:
- Growth factor
- Growth factor receptor
- Protein involves in signal transduction
- Nuclear regulatory protein
- Cell cycle regulator
Abnormal function of oncogene
1. It May encode growth factors that stimulate the tumor by an autocrine mechanism.
Example:
- HST over expression → Stomach cancer
- HGF overexpression → Hepatocellular carcinoma.
- FGF Amplification → Bladder cancer, breast cancer
2. It May encode growth factor receptors, thus increasing the number of receptors on the tumor cells.
Example:
- ERBB1 → Mutation → Adenocarcinoma of the lung
- ERBB2 Amplification → Breast cancer
- PDGFRB → over expression → Glioma, Leukemia
3. May encode growth defective signal transduction that transmits growth-promoting signal without an external trigger.
Example:
- KRAS → point mutation → Colon, lung, and pancreatic tumor.
- ERBB2 → Amplification → Breast cancer
- ABL → Translocation → CML
4. It May encode a transcription factor that binds to DNA and stimulates cell growth.
Example:
- C-myc → Translocation → Burkitt lymphoma.
- N-myc → Amplification → Neuroblastoma
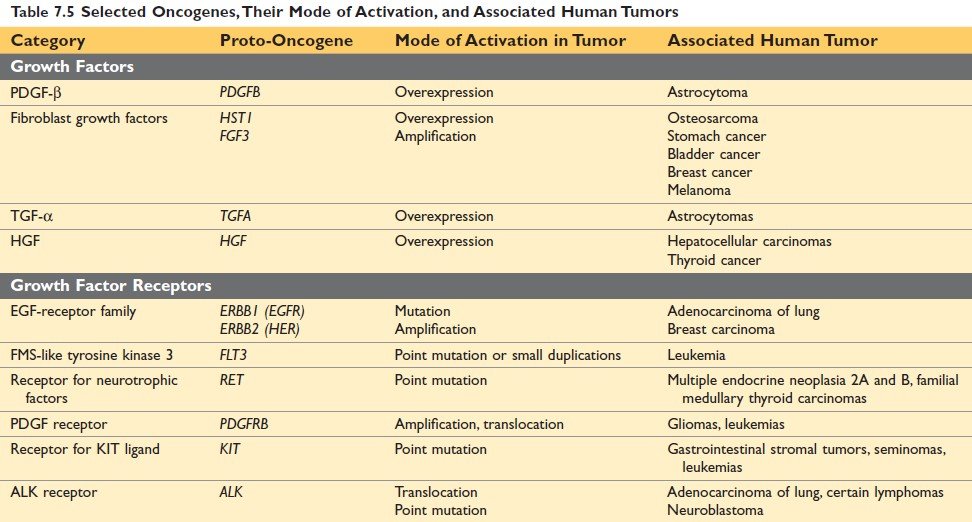
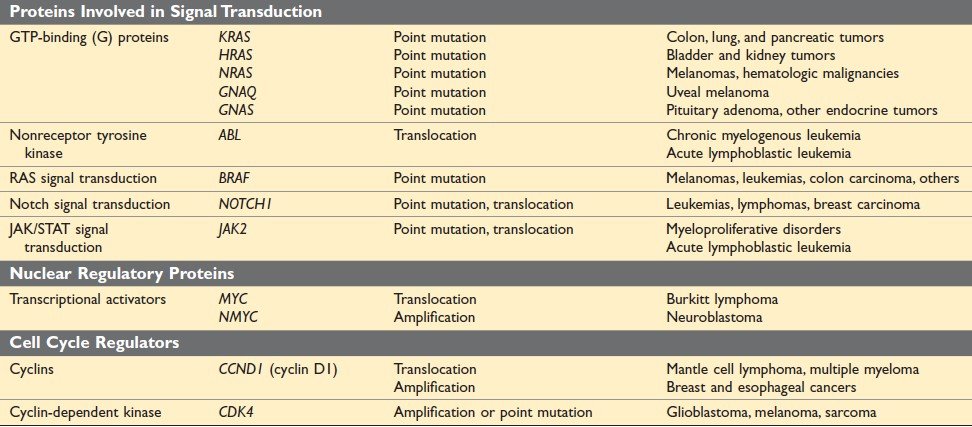
Figure: Selected Oncogenes, Their Mode of Activation, and Associated Human Tumors (Ref Robbin’s Page 285)
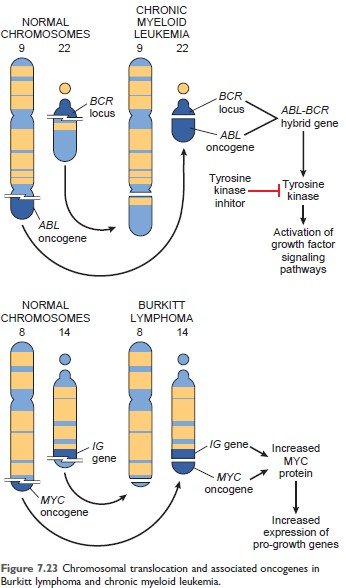
Figure: Chromosomal translocation and associated oncogenes in Chronic myeloid leukemia (Robbin’s 10th Page 288)
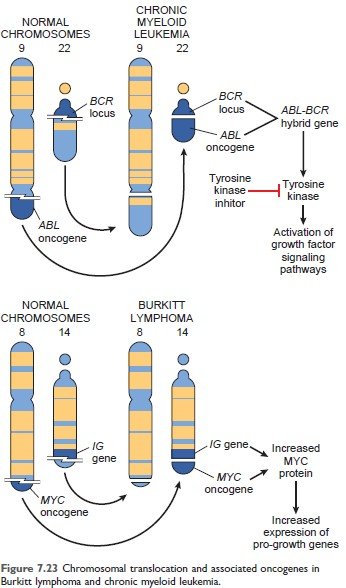
Figure: Chromosomal translocation and associated oncogenes in Burkitt lymphoma (Robbin’s 10th Page 288)
B. Cancer suppressor gene:
The term "tumor suppressor genes" is a misnomer because the physiologic function of these genes is to regulate cell growth (ie. inhibit normal cell growth), not to prevent tumor formation.
Abnormality of these genes leads to failure of growth inhibition.
Selected tumor suppressor gene:
| Gene | Normal function | Associated cancer |
|
APC
|
Inhibition of signal transduction
|
Ca. of stomach, colon, pancreas
|
|
RB
|
Inhibition of G1/S transition during the cell cycle
|
Retinoblastoma
|
|
TP53
|
Cell cycle arrest and apoptosis in response to DNA damage
|
Most human cancer
|
|
BRCA 1, BRCA 2
|
Repair double-strand break in DNA
|
Breast and ovarian cancer
|
|
NF1
|
Inhibition of RAS signaling
|
Neurofibromatosis
|
C. Apoptosis regulatory gene:
- Mutation of this gene is also involved in tumor genesis.
- Bcl-2 protects tumor cells from apoptosis.
- Overexpression of Bcl-2 extends the survival of genetically damaged cells and neoplastic proliferation.
D. DNA repairing gene:
- They correct errors in DNA that occur, spontaneously during cell division, or following exposure to mutagenic chemicals or irradiation.
- Mutation results in incorrection of DNA repair genes and the proliferation of defective cells.
- Example: Xeroderma pigmentosum.
Growth of transformed cells
The formation of a tumor mass is influenced by:
- Kinetics of tumor cell growth
- Tumor angiogenesis
- Tumor progression and heterogeneity
1. Kinetics:
The rate of growth of a tumor is determined by three main factors:
- the doubling time of tumor cells,
- the fraction of tumor cells that are in the replicative pool, and
- the rate at which cells are shed or die.
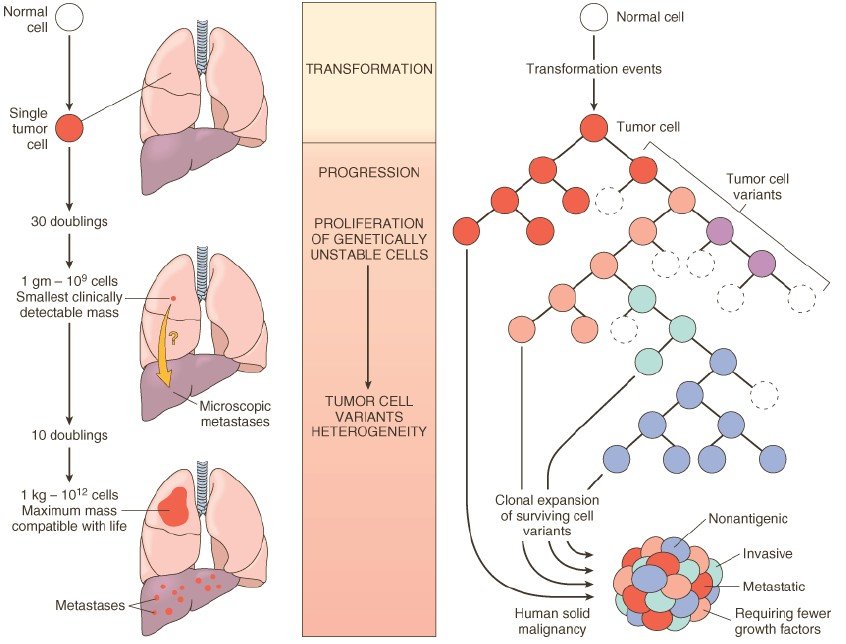
Figure: Left: Biology of tumor growth. Right: Tumor progression and generation of heterogeneity. (Robbin’s 7th Edition)
2. Tumor angiogenesis:
Because tumor cells need oxygen to survive, the vascularization of tumors by host-derived blood vessels has a profound influence on tumor growth. Vascularization is affected by the release of tumor-associated angiogenic factors derived from tumor cells.
- Vascular endothelial growth factor,
- Basic fibroblast growth factor,
- Angiostatin,
- Endostatin and vasculostatin
3. Tumor progression and heterogeneity:
Tumor progression is a phenomenon whereby tumors become progressively more aggressive and acquire greater malignant potential.
Example: Preneoplastic lesions → carcinoma in situ → invasive cancer.
Heterogeneity:
Clinically detectable tumor, although monoclonal origin, is usually made of phenotypically and genetically heterogenous cell. This is called heterogeneity.
During the growth of the tumor, there is the sequential appearance of subpopulations of cells having different characteristics, such as,
- Karyotypic change,
- Invasiveness,
- Rate of growth
- Hormone responsiveness,
- Susceptibility to anticancer therapy
- Metastatic potentiality
Molecular basis of multistep carcinogenesis:
The concept of “multistep carcinogenesis’’ provided by the study of oncogenes and tumor suppressor genes. Most human cancers have multiple genetic alterations involving the activation of several oncogenes and the loss of two or more tumor suppressor genes.
Example Molecular basis of multistep carcinogenesis
The study of colon carcinoma progresses through a series of:
A) Morphologically identifiable stages:
Colon epithelial hyperplasia is followed by the formation of adenomas that progressively enlarge and ultimately undergo malignant transformation.
B) Molecular changes include, the inactivation of the APC tumor suppressor gene occurring first, followed by activation of RAS, loss of genes on 18q, and ultimately, loss of p53 and TGF-13 receptor 11 genes.
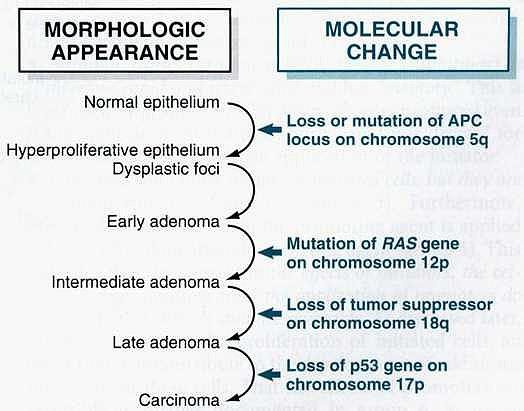
Figure: Molecular model for the evolution of colorectal cancers through the adenoma-carcinoma sequence.
Mechanism of invasion and metastasis:
Invasion and metastasis are biological hallmarks of malignancy. Metastasis can be divided into two phases:
- Invasion of the extracellular matrix
- Vascular dissemination and homing of tumor cells.
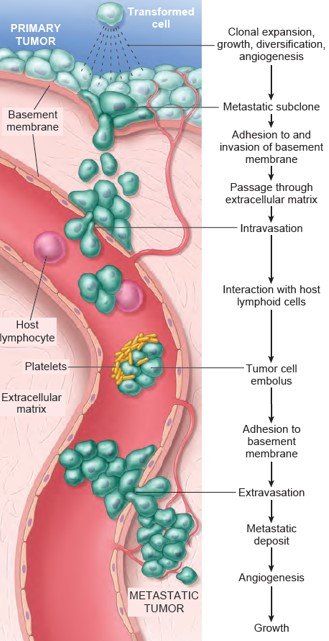
Figure: The metastatic cascade. Sequential steps are involved in the hematogenous spread of a tumor. (Robbin’s 10th Edition Page: 306)
1. Invasion of extracellular matrix:
- Detachment of tumor cells from each other
- Attachment to matrix component
- Degradation of ECM
- Migration of tumor cells
A. Detachment of tumor cells from each other:
The tumor cells remain attached to each other by several adhesion molecules. In several carcinomas, there is a down-regulation of Adhesion molecules, reducing the cohesiveness of tumor cells. This facilitates their detachment from the primary tumor.
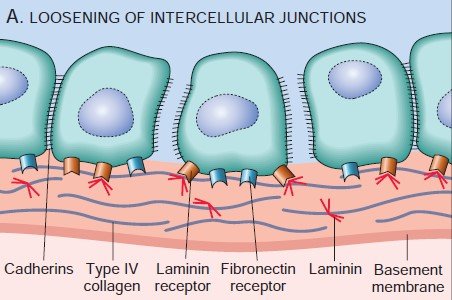
Figure: Detachment of tumor cells from each other
B. Attachment to matrix component
To penetrate the surrounding ECM, the tumor cells must first adhere to the matrix components. The Tumor cells attached to Laminin and Fibronectin receptor is important for invasion and metastasis.
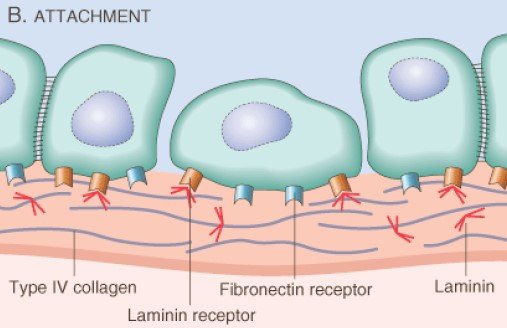
Figure: Attachment to matrix component
C. Degradation of ECM:
Proteolytic enzymes (Type-IV Collagenase, Cathepsin D, Urokinase-type Plasminogen Activator) secreted by tumor cells degrade the matrix components (laminin, fibronectin and Proteoglycans) and create a passageway for migration.
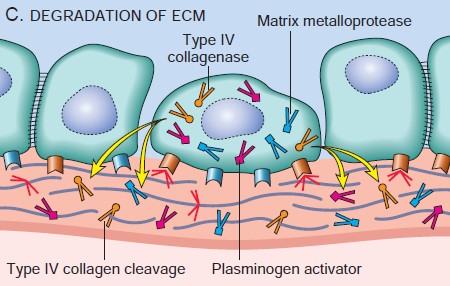
Figure: Degradation of ECM
D. Migration of tumor cells
Migration is mediated by tumor cell-derived autocrine motility factor. In addition, cleavage Products of matrix components and some growth factors have chemotactic activity for tumor cells.
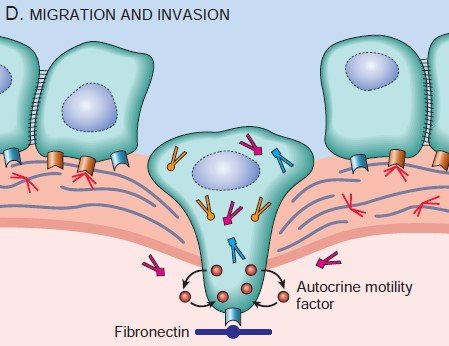
Figure: Migration of tumor cells
2. Vascular dissemination and homing of tumor cells:
Within the circulation, tumor cells tend to aggregate in clumps. Adhesion of tumor cells occurs with two types of cells.
- Homotype (between tumor cells)
- Heterotype (between tumor cells and blood cells, particularly platelets)
The formation of platelet-tumor aggregates enhances tumor cell survival and implantability. Extravasations of tumor emboli at distant sites involve adhesion to endothelium, followed by migration through the basement membrane.
The sites where tumor cells leave the capillaries to form secondary deposits are related to the:
1. Anatomic location and vascular drainage of the primary tumor.
2. Tropism of the particular tumors for specific tissues.
For example:
- Prostatic carcinoma spread to the bone,
- Bronchogenic carcinoma tends to involve the adrenals and brain
- Neuroblastomas spread to the liver and bone.
Such organ tropism may be related to:
- Adhesion molecules of tumor cells may have whose ligands are expressed on the endothelial cells of the target organ.
- Chemokines have an important role in determining target tissue for metastasis. For example, some breast cancer cells express the chemokine receptors CXCR4 and CCR7.
- The microenvironment of the organ or site. Although well vascularized, skeletal muscle and spleen are rarely sites of metastasis.
Carcinogen:
The agents, which produce genetic damage and induce neoplastic transformation are called carcinogens. They are three types of carcinogens:
- Chemical carcinogen
- Radiation and
- Oncogenic microorganism.
Steps involved in chemical carcinogenesis:
- Initiation
- Promotion
A. Initiation:
Initiation results from exposure of cells to a sufficient dose of a carcinogenic agent (initiator);
- Initiation causes permanent DNA damage (mutations).
- It is rapid and irreversible and has "memory.
- Initiation alone is not sufficient for tumor formation (group-1)
B. Promoter:
They induce tumors in initiated cells, but they are nontumorigenic by themselves.
- Do not affect DNA directly and are reversible.
- Tumors do not result when the promoting agent is applied before, rather than after the initiating agent (group-4).
Interpretation:
- Only initiator no promotor → no tumor
- First initiator then application of promotor (twice weekly interval) → tumor
- First initiator then after a long time application of promotor → tumor
- First promotor then initiator → no tumor
- Only Promotor → no tumor
- First initiator than the application of promotor at monthly intervals → no tumor.
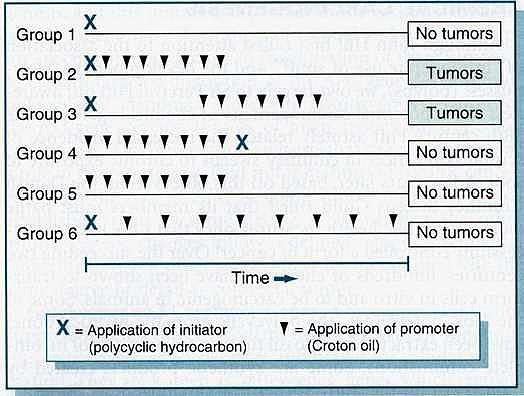
Figure: Initiation and promotion phases of carcinogenesis in mice.
Carcinogen:
- Complete carcinogens - the chemical carcinogens that have the capability of initiation and promotion are called complete carcinogens.
- Incomplete carcinogen - which has only the capability of initiation.
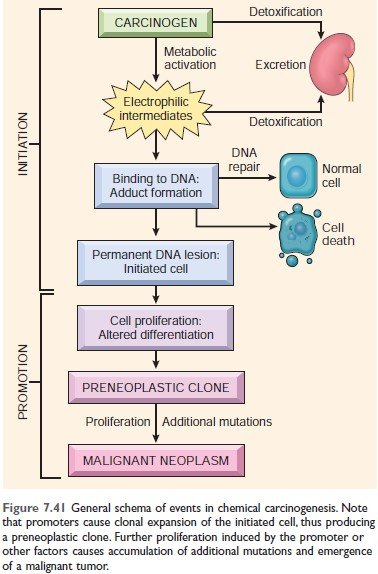
Figure: Events in chemical carcinogenesis. (Robbin’s 10th Edition Page 321)
Initiation of Chemical carcinogen falls into two categories:
- Direct acting carcinogen: require no metabolic conversion to become carcinogenic.
- Indirect acting or pro-carcinogen: requires metabolic conversion in the body to become the ultimate carcinogen.
A. Major chemical carcinogen:
|
A) Direct-acting carcinogen: 1) Alkylating agents
2) Acylating agents
|
|
B) Indirect-acting carcinogen: 1) Polycyclic and Heterocyclic aromatic hydrocarbon
2) Aromatic amine, amides, and Azo dyes
3) Natural Plant and Microbial Products
4) Others
|
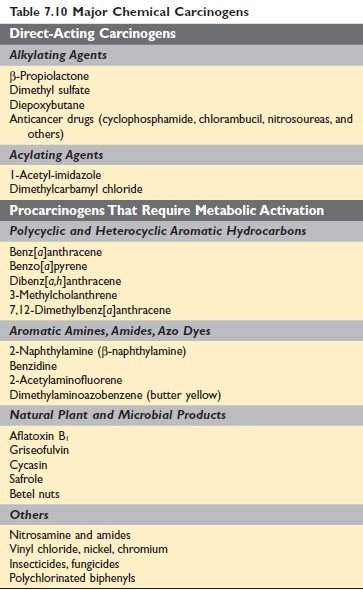
Figure: Major chemical carcinogens Robbin’s 10th Edition Page 321
Promotion of Chemical Carcinogenesis
The carcinogenicity of some chemicals is augmented by the subsequent administration of promoters.
- Phorbol esters, hormones, phenols, and drugs.
B. Radiation carcinogen:
- Ultraviolet Rays: UVA, UVB, UVC
- Ionizing Radiation:
- Electromagnetic: x-ray, γ-ray and
- Particulate: α-particles, β-particles, protons, neutrons
Ultraviolet Rays:
UV rays are of three types:
- UVA- 320-400 nm wavelengths
- UVB-280-320 nm wavelength- the possible culprit
- UVC- 200-280 nm wavelength- filtered by the ozone layer.
Mechanism of UV rays: The carcinogenicity of UVB light is due to the formation of pyrimidine dimers in DNA
Associated cancer: Squamous cell carcinoma, basal cell carcinoma, and malignant melanoma of the skin
Effect of UV rays:
- They may inhibit cell division
- Inactivation of enzyme
- May cause mutagenic effect of DNA
- May kill the cell in a sufficient dose
Ionizing Radiation:
Common radiation-induced cancers are:
- Myeloid Leukaemia
- Thyroid cancer
- Breast, lung, salivary glands
Mechanism: Causes chromosome breakage, translocation, and less frequently, point mutations, leading to genetic damage and carcinogenesis.
Examples:
- Fanconi’s anemia
- Ataxia talengiectaisa
- Bloom’s syndrome
- Xeroderma pigmentosum.
C. Oncogenic microorganism
A) Oncogenic virus:
|
I) DNA virus: 1. Human papilloma virus (HPV)
2. Hepatitis-B virus:
3. Epstein-Barr virus:
|
|
II) RNA virus: Human T-cell Leukemia Virus Type-1 (HTLV-1):
|
B. Oncogenic bacteria
Helicobactor pylori: Gastric lymphoma and gastric carcinoma
Effect of tumor on host
- Local effect
- Hormonal effect
- Cancer cachexia
- Paraneoplastic syndrome
Local effect
- Pituitary adenoma: Although benign but expansile growth can destroy pituitary- serious endocrine insufficiency
- Cancer in the endocrine gland: Endocrine insufficiency
- Neoplasm in the gut: Cause obstruction
Hormonal effect
Well-differentiated tumors (usually benign tumors) have functional capacity.
Example:
- β- cell adenoma of pancreatic islet cells produces increased insulin: Hypoglycemia
- Paraneoplastic syndrome: Non-endocrine tumors may produce Ectopic hormones.
- Small cell carcinoma of the lung: ACTH (Cushing syndrome)
Bleeding and secondary infection
Tumors of the skin or mucosa of GIT or urinary tract cause - Ulceration, secondary infection, and bleeding (melena, haematuria)
Cancer cachexia
Patients with cancer suffer from progressive loss of body fat and lean body mass, resulting in weakness, anorexia, and anemia. This wasting syndrome is called cachexia.
Cause: Action of cytokine (TNF, IL-1, IFN-γ) from tumor cells and host cells in response to the tumor.
Paraneoplastic syndrome:
Symptom complexes in cancer-bearing patients that cannot readily be explained, either by the local or distant spread of tumor or by the elaboration of hormones indigenous to the tissue from which the tumor arose, are known as paraneoplastic syndromes.
Importance of Paraneoplastic syndrome:
- May represent the earliest manifestation of an occult neoplasm.
- May represent significant clinical problems and may even be lethal.
- May mimic metastatic disease and therefore confound treatment.
Common clinical syndromes:
- Endocrinopathies
- Nerve and muscle syndrome
- Dermatologic disorder
- Osseous, articular, and soft tissue changes
- Vascular and hematologic changes
|
Clinical syndrome
|
Cancer
|
Mechanism
|
|
Cushing syndrome
|
Small cell carcinoma of the lung, Pancreatic ca, Neural tumor
|
ACTH or ACTH-like substance
|
|
Syndrome of inappropriate ADH secretion
|
Small cell carcinoma of the lung
|
ADH
|
|
Hypercalcemia
|
SCC of the lung, Breast ca, Renal ca, Ovarian ca
|
Parathyroid hormone-related protein
|
|
Hypoglycemia
|
Fibrosarcoma, HCC
|
Insulin
|
|
Polycythemia
|
Renal ca, HCC, Cerebellar hemangioma
|
Erythropoietin
|
|
Carcinoid syndrome
|
Bronchial adenoma, Pancreatic ca, gastric ca
|
Serotonin, Bradykinin
|
|
Clinical syndrome
|
Cancer
|
Mechanism
|
| Myasthenia | Bronchogenic carcinoma | Immunologic |
|
Clinical syndrome
|
Cancer
|
Mechanism
|
|
Acanthosis nigricans
|
Gastric carcinoma,
Lung carcinoma |
Immunologic
|
|
Dermatomyositis
|
Bronchogenic carcinoma
Breast carcinoma |
Immunologic
|
|
Clinical syndrome
|
Cancer
|
Mechanism
|
|
Hypertrophic osteoarthropathy
& clubbing of fingers
|
Bronchogenic carcinoma
|
Unknown
|
|
Clinical syndrome
|
Cancer
|
Mechanism
|
|
Venous thrombosis
(Trousseau phenomenon)
|
Bronchogenic carcinoma
|
Tumor product (mucin)
|
|
NBTE
|
Advanced cancers
|
Hypercoagulability
|
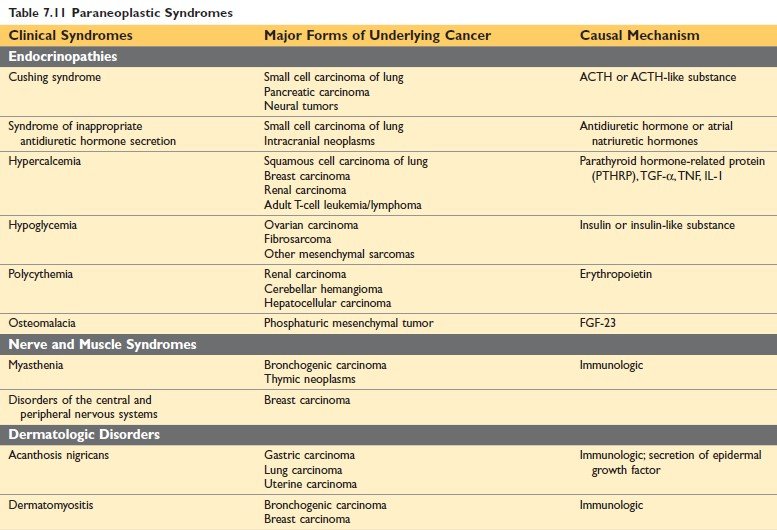
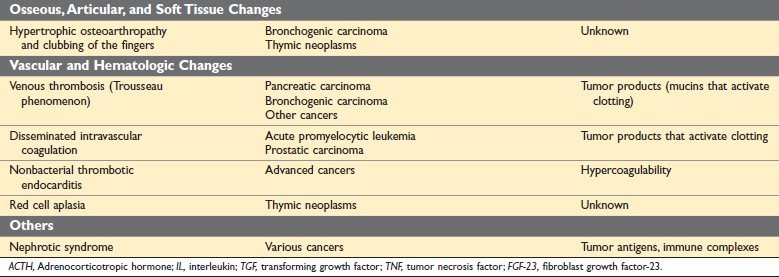
Figure: Paraneoplastic Syndrome (Robbin’s 10th Edition Page 329)
Grading of cancer:
Grading means the level of differentiation.
Grading of cancer is based on the:
- Degree of differentiation and
- The number of mitosis within the tumor.
Cancer is classified in Grade I to IV with increasing anaplasia.
- Grade-I: > 75% of cells are differentiated.
- Grade-II: > 50% of cells are differentiated.
- Grade- III: >25% of cells are differentiated.
- Grade- IV: <25% of cells are differentiated.
Staging of cancer:
Staging:
Staging means the extent of the spread of cancer within the patient. Staging is based on the presence or absence of blood-borne metastasis.
Staging system:
- UICC (Union Internationale Contre Cancer): employs a TNM system
- AJC (American Joint Committee): employs stages 0 - IV.
TNM system
|
T: Size of the tumor
|
|
N: regional lymph node involvement
|
|
M: distant metastasis
|
Importance of Staging:
Staging is more important clinically, to select the best form of therapy for the patient.
Laboratory diagnosis of tumor:
- Cytologic and Histologic methods
- Immunohistochemistry
- Molecular diagnosis
- Flow cytometry
- Tumor markers
A. Cytological examination:
- Study of the cell.
- Stain: Papanicolaou stain
Types of Cytological examination:
- Exfoliative cytology
- Abrasive cytology
- Fine needle aspiration cytology (FNAC)
1. Exfoliative cytology:
Collection of exfoliated cells from the body. Such as,
- Ascitic fluid: carcinoma of stomach, kidney, liver
- Pleural fluid: carcinoma of the lung
- CSF: CNS tumor
- Urine: carcinoma of the urinary bladder
- Synovial fluid: Bone tumor
- Sputum: Bronchogenic carcinoma
- Gastric lavage: Carcinoma of the stomach
Figure: Malignant cell in Pap stain
2. Abrasive cytology
- Pap’s smear: Carcinoma of cervix, prostate, endometrial carcinoma
- Bronchial brush cytology
- Scraping of the ulcerated lesion
3. Fine needle aspiration cytology (FNAC)
The procedure involves aspiration cells and attendant fluid with a small-bore needle, followed by cytologic examination of the stained smear.
Use:
- Most commonly for assessment of readily palpable lesions (ex: thyroid, lymph node, breast).
- Deep-seated structure by guidance from imaging (USG, CT, ex liver kidney, lung, pancreas, pelvic lymph nodes)
Importance:
- Less invasive and more rapidly performed than needle biopsies
- Obviates surgery and its attendant risks.
- May be confounded by sampling errors, in experienced hands, it is rapid and quite reliable.
Histopathological examination:
- Study of tissue.
- Stain - Hematoxylin, and Eosin (H&E) stain.
- The specimen must be:
- Adequate
- Representative
- Properly preserved in fixative (10% formalin).
(It prevents autolysis e.g. preserve tissue architecture by protein denaturation.)
B. Immunohistochemistry:
Immunohistochemistry involves the detection of cell products or surface markers by monoclonal antibodies.
Principle: The binding of antibodies can be revealed by fluorescent labels or chemical reactions that result in the generation of colored products.
Examples of Utility of immunohistology:
1. Categorization of the undifferentiated malignant tumor by the tissue-specific intermediates.
Intermediate filaments and their distribution:
| Keratin | Carcinoma, mesothelioma, points to an epithelial origin |
| Desmin | Muscle tumor |
| Vimentin | Mesenchymal tumor, some carcinoma |
| Glial filaments | Gliomatous tumors |
| Neurofilaments | Neuronal tumor |
2. Categorization of leukemia and lymphomas by using monoclonal antibodies specific for various lymphohematopoietic cells.
3. Determination of the primary site of metastatic tumors by using reagents that identify specific cell types. Example- thyroglobulin for thyroid cancer, Prostate-specific antigen for prostate cancer.
4. Detection of molecules that have prognostic or therapeutic significance. Example - detection of estrogen /progesterone receptor of breast cancer.
C. Molecular diagnosis:
By Southern blot analysis, polymerase chain reaction (PCR).
- Diagnosis of malignant neoplasms
- Prognosis of malignant neoplasm
- Detection of minimal residual disease
- Diagnosis of hereditary predisposition to cancer
- Guiding therapy with oncoprotein-directed drugs
- Identifying mechanisms of drug resistance: liquid biopsies.
D. Flow cytometry:
- Measurement of the DNA content of tumor cells.
- Detection of cell surface antigen in leukemia and lymphoma
- Detection of ploidy.
An advantage of flow cytometry over immunohistochemistry is that multiple antigens are assessed simultaneously on individual cells using combinations of specific antibodies linked to different fluorescent dyes.
E. Tumor marker:
Tumor markers are the biochemical indicator of the presence of a tumor.
Importance:
- To support the diagnosis
- To see the response to therapy
- To see the relapse during the follow-up period.
Example of Tumor marker:
1) Hormones
| Tumor Marker | Tumor Types |
| Human chorionic gonadotropin | Trophoblastic tumor, Non-seminomatous testicular germ cell tumor |
| Calcitonin | Medullary carcinoma of the thyroid |
| Catecholamines and metabolites | Pheochromocytoma |
2) Oncofetal antigen
| α- fetoprotein | Hepatocellular carcinoma, Non-seminomatous testicular tumor |
| Carcinoembryonic antigen | Carcinoma of the colon, breast, lung, stomach, pancreas, and heart |
3) Lineage-Specific Proteins
| Immunoglobulin | Multiple myeloma and other gammopathies |
| Prostate-specific antigen and prostate-specific membrane antigen | Prostate cancer |
| Prostatic acid phosphatase | Prostate cancer |
| CA-125 | Ovarian cancer |
| CA-19-9 | Pancreatic cancer, colon cancer |
| CA-15-3 | Breast cancer |
| EGFR mutants in serum | Lung cancer |
| TP53, APC, RAS mutation in stool and serum | Colon cancer |
| TP53, RAS mutation in stool and serum | Pancreatic cancer |
| TP53, RAS mutation in sputum and serum | Lung cancer |
| TP53 mutation in urine | Bladder cancer |
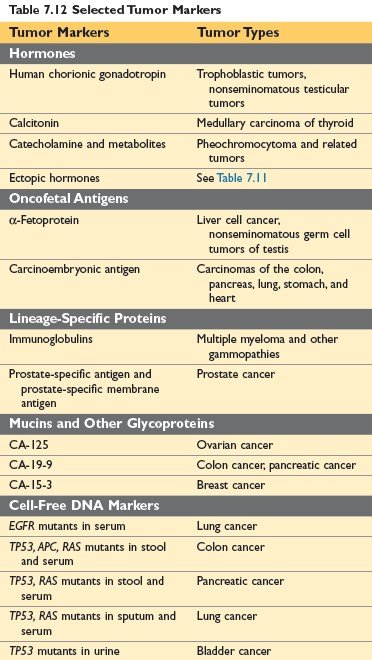
Figure: Selected Tumor Markers (Robbin’s 10th edition Page 336)

Comments (0)