CHRONIC INFLAMMATION:
Chronic inflammation can be defined as, inflammation of prolonged duration (weeks or months) in which active inflammation, tissue destruction, and attempts at repair coexist, in varying combinations.
Morphologic feature of chronic inflammation:
In contrast to acute inflammation, which is manifested by vascular changes, edema, and predominantly neutrophilic infiltration, chronic inflammation is characterized by the following:
- Infiltration with mononuclear cells, including macrophages, lymphocytes, and plasma cells.
- Tissue destruction is induced by the persistent offending agent or by the inflammatory cells.
- Attempts at healing by connective tissue replacement, accomplished by angiogenesis and fibrosis.
Causes of chronic inflammation:
1. Persistent microbial infections that are difficult to eradicate
- Mycobacterium (tuberculosis)
- Treponema pallidum (Syphilis)
- Certain viruses, fungi, and parasite
2. Prolonged exposure to potentially toxic agents
- Silicosis (inflammatory lung diseases due to silica)
- Atherosclerosis
3. Hypersensitivity diseases
- Autoimmune diseases: Rheumatoid arthritis, Lupus erythematosus.
- Allergic diseases: Bronchial asthma
Role of macrophages in Chronic Inflammation:
The products of activated macrophages serve to eliminate injurious agents such as microbes and to initiate the process of repair and are responsible for much of the tissue injury in chronic inflammation.
The dominant cells in most chronic inflammation are macrophages, which contribute by
- Phagocytosis
- Initiate repair and involved in scar formation and fibrosis (growth factors)
- Initiation and propagation of inflammatory reactions (secrete cytokines and eicosanoids).
- Act as antigen-presenting cell and participate in cell-mediated immunity
Nice to Know:
|
Macrophages are normally diffusely scattered in most connective tissues.
Together, these cells comprise the Mononuclear Phagocyte System, also known by the older (and inaccurate) name Reticuloendothelial system. Committed progenitors in the bone marrow give rise to monocytes, which enter the blood, migrate into various tissues, and differentiate into macrophages. |
Types of chronic inflammation
1. Specific Chronic Inflammation:
Histologically distinctive
Example: Granulomatous Inflammation
2. Non-specific Chronic Inflammation:
Not histologically distinctive
Example: Peptic ulcer, ulcerative colitis
Granulomatous inflammation:
Granulomatous inflammation is a form of chronic inflammation characterized by collections of activated macrophages, often with T lymphocytes, and sometimes associated with necrosis.
Example of Diseases with Granulomatous Inflammation:
| Disease | Cause | Tissue reaction |
| Tuberculosis | Mycobacterium tuberculosis | Caseating granuloma (tubercle): Focus of activated macrophages (epithelioid cells), rimmed by fibroblasts, lymphocytes, histiocytes, and occasional Langhans giant cells; Central caseous necrosis with amorphous granular debris; Acid-fast bacilli |
| Leprosy | Mycobacterium leprae | Non-caseating granuloma |
| Syphilis | Treponema pallidum |
Gumma: microscopic to grossly visible lesion, enclosing wall of histiocytes; plasma cell infiltrate; central cells are necrotic without loss of cellular outline
|
| Cat-scratch disease | Gram negative bacillus |
Rounded or stellate granuloma
|
| Sarcoidosis | Unknown etiology |
Non-caseating granuloma
|
| Crohn's disease (Inflammatory Bowel Disease - IBD) | Immune reaction against intestinal bacteria, possibly self-antigens |
Occasional Non-caseating granuloma in the wall of the intestine, with dense chronic inflammatory infiltrate.
|
|
Amorphous means without clearly defined shape/form Caseating granuloma in TB is referred to as tubercle (soft tubercle). It is often characterized by central caseous necrosis; where there is the total loss of cellular architecture & cell details (amorphous granular appearance) In Gumma – Central Coagulative necrosis occurs; where cellular shape, outline & architecture are preserved. |
Classification of Granulomatous reaction:
Morphologically or (Morphological classification of Granuloma)
1. Diffuse granulomatous reaction: Lepromatous leprosy
2. Tuberculoid granulomatous reaction:
- Non-caseating tuberculoid reaction: Sarcoidosis, Crohn’s disease, lupus vulgaris and Tuberculoid leprosy.
- Caseating tuberculoid reaction: Tuberculosis
3. Suppurative tuberculoid reaction: (Associated with pus formation): Cat-scratch disease, Lymphogranuloma venereum
Granuloma:
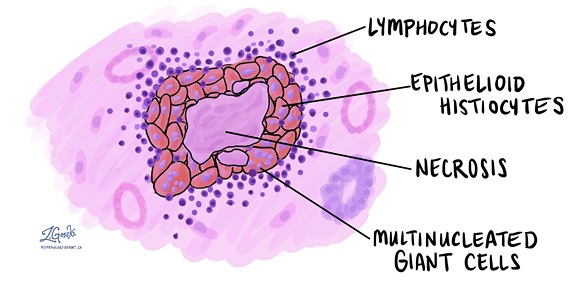
A granuloma is a focus of chronic inflammation consisting of a microscopic aggregation of macrophages that are transformed into epithelial-like cells surrounded by a collar of mononuclear leukocytes, principally lymphocytes and occasionally plasma cells.
Granuloma formation is a cellular attempt to contain an offending agent that is difficult to eradicate. In this attempt, there is often strong activation of T lymphocytes leading to macrophage activation, which can cause injury to normal tissues.
The activated macrophages may develop abundant cytoplasm and begin to resemble epithelial cells and are called epithelioid cells. Some activated macrophages may fuse, forming multinucleate giant cells.
Classification of Granuloma:
A) On the basis of pathogenesis:
1. Foreign body granulomas: incited by relatively inert foreign bodies which were resistant to digestion/degradation. And induce inflammation in the absence of T cell–mediated immune responses. Eg: due to talc (associated with intravenous drug abuse), sutures, or other fibers that are large enough to preclude phagocytosis by a macrophage and are not immunogenic.
2. Immune granuloma: caused by insoluble particles, typically persistent microbes (i.e difficult to eradicate)
are caused by a variety of agents that are capable of inducing a persistent T cell-mediated immune response. In such responses, activated Th1 cells produce cytokines such as IFN-γ, which activate macrophages.
B) Histological classification:
1. Non-caseating granuloma:
No central region of necrosis
Found in inflammatory conditions (e.g., sarcoidosis and Crohn's disease), vasculitis, and exposure to foreign objects.
2. Caseating granuloma:
Here, the Central region of necrosis is present.
Commonly found in Immune granuloma
C) According to etiology
- Bacterial
- Fungal
- Parasitic
- Miscellaneous
Epithelioid cells are the specialized macrophages
- 15-30 micron in diameter
- Having pale pink granular cytoplasm with indistinct cell boundaries
- The nucleus is oval or elongated
Multinucleated Giant Cells (found in granuloma)
- Frequently formed by the fusion of epithelioid cells. (Diameter: 40-50 μm),
- Have a large mass of cytoplasm, containing 20 or more nuclei,
- Arranged peripherally (Langhans type giant cell) or haphazardly throughout the cytoplasm (Foreign body giant cell)
Giant cell:
Giant cells are large cells with more than one nucleus.
Types of giant cells
A. Physiological:
- Osteoclast in bone
- Syncytotrophoblast of placenta
- Megakaryocytes in bone marrow
B. Pathological:
- Fused macrophage:
- Foreign body giant cell (in Foreign body granuloma)
- Langhans’ giant cell (in Tuberculous granuloma)
- Aschoff’s giant cell
- Reaction to insoluble material: sodium urate crystal, keratin, fat
- Fused cell due to viral infection:
- Epithelial giant cell in herpes virus infection
- Warthin-Finkeldey giant cell in measles.
- Tumor giant cells: found in-
- Giant cell tumors of bone,
- Choriocarcinoma
- Reed-Sternberg cell in Hodgkin’s diseases
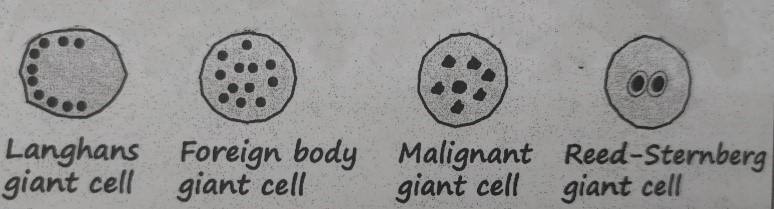
Figure: Giant Cells
Morphology/Histological finding of Caseating granuloma (tubercle):
- Central necrosis with amorphous granular debris
- Focus of activated macrophages (epithelioid cells), rimmed by fibroblasts, lymphocytes, histiocytes,
- Occasional Langhans giant cells (Epithelioid cells fuse to form giant cells peripherally).
Morphology of granuloma:
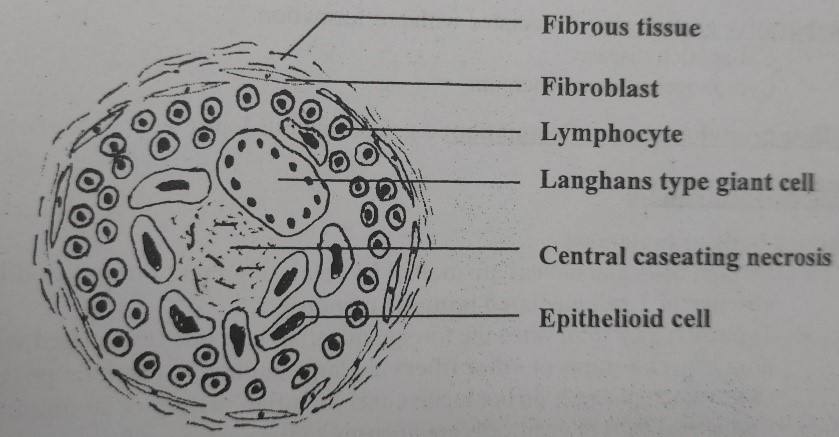
Figure: Granuloma/TB Granuloma/ Soft tubercle Granuloma/ Immune Granuloma
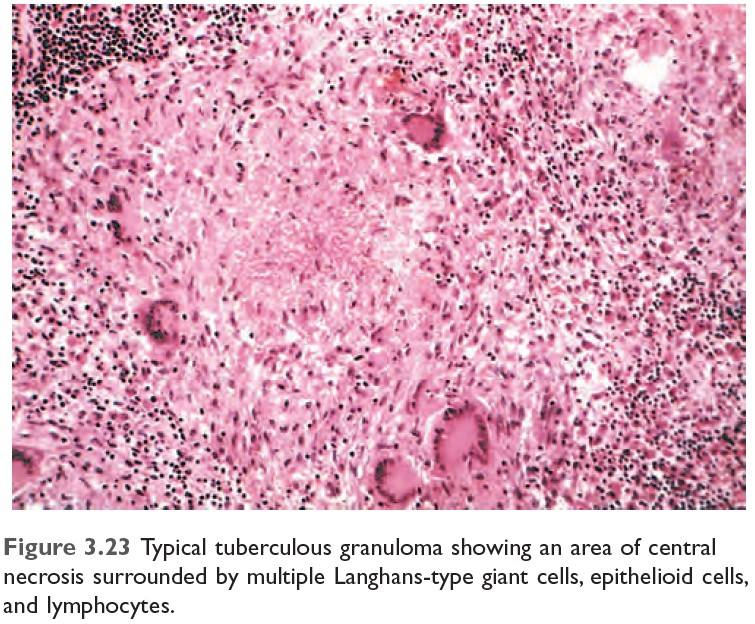
Figure: Typical TB Granuloma
Figure: Summary of Chronic Inflammation
TB Granuloma/Immune Granuloma Formation Mechanism: ***
|
Macrophages engulf material
↓ And it presents antigen to naïve CD4 T cell & macrophage secrete IL-12 and T cell is differentiated to Th1 cell (Type 1 T helper cell) ↓ Activated Th1 cell secretes:
↓
Activated macrophage promotes ↓ T cell stimulation by more antigen presentation & via cytokines (such as IL-12) ↓ Promote monocyte recruitment by secreting TNF, chemokines ↓ Prolonged reaction involving T cell & macrophage induce more & more macrophages activation & macrophage activated by IFN-γ differentiate into epithelioid cells & multinucleated giant cells → Formation of granuloma |
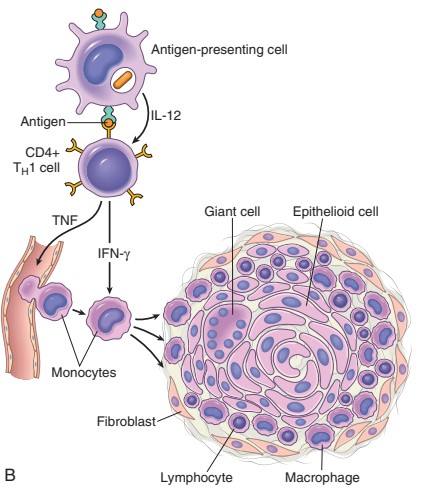
Figure: Mechanism of Granuloma Formation (Source: Robbin’s 9th Edition)
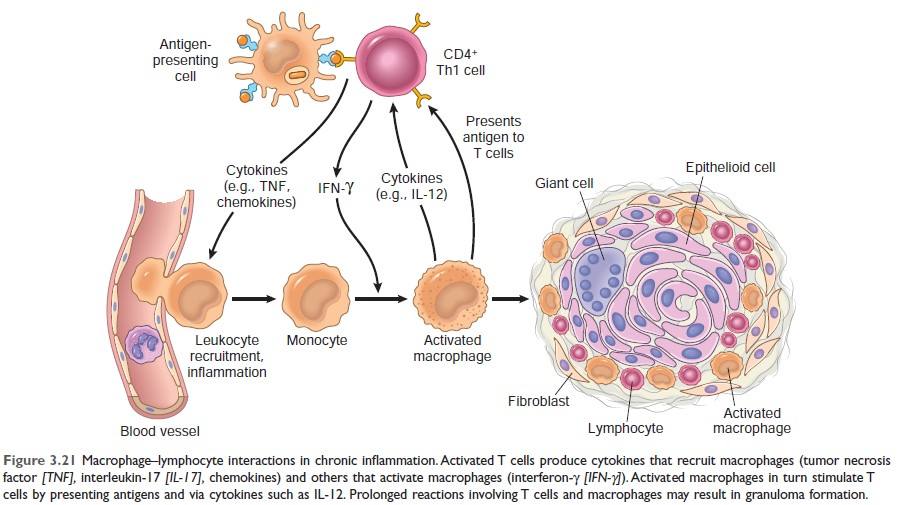
Figure: Mechanism of Granuloma Formation (Source: Robbin’s 10th Edition Page:99)
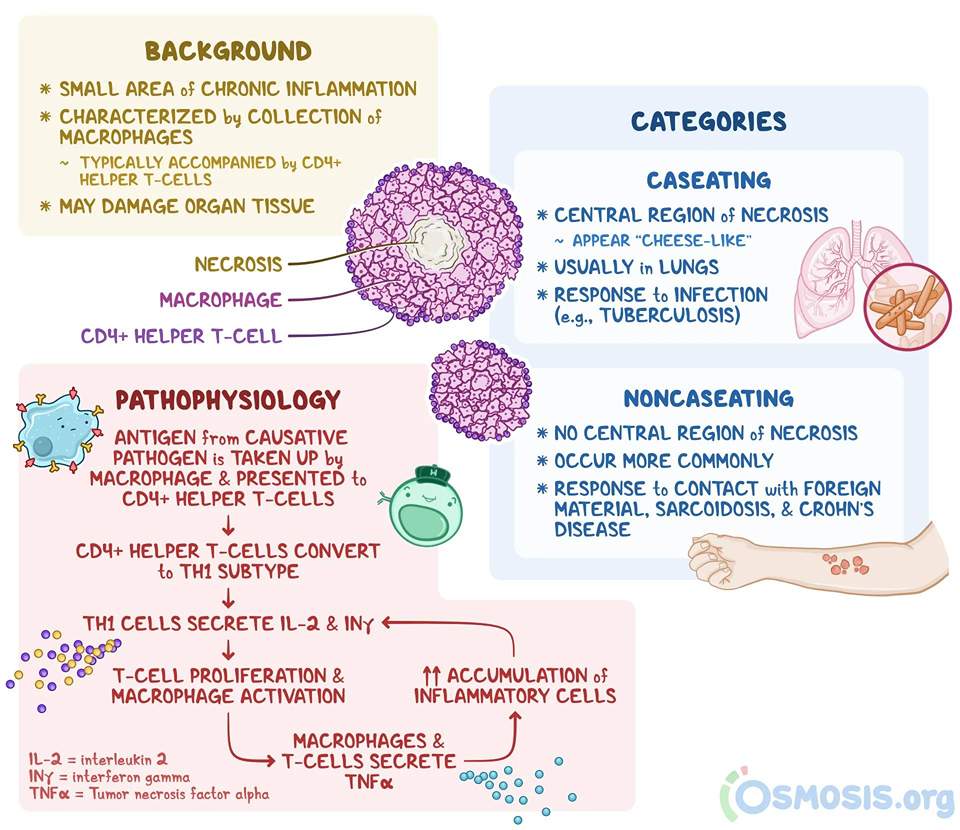
Figure: Oveview of granuloma formation (Ref Osmosis.org)
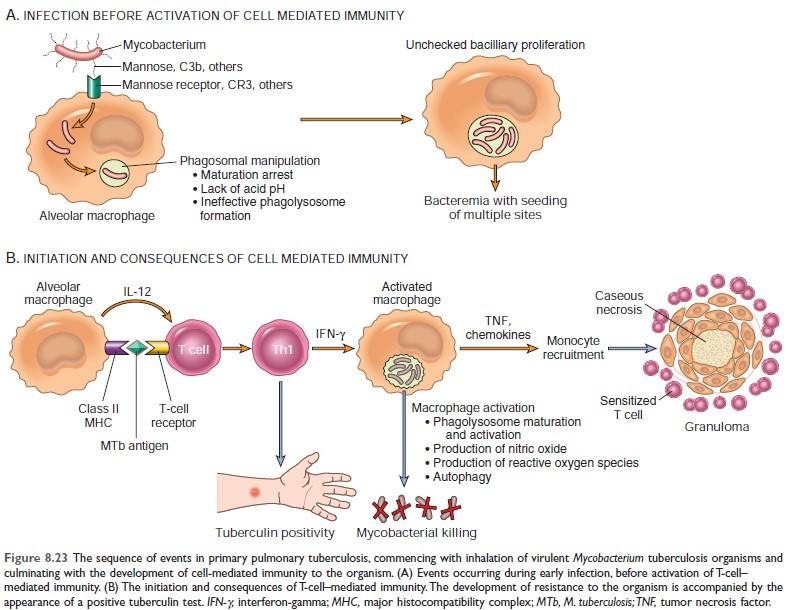
Figure: Mechanism of granuloma formation in TB [Source: Robbin’s 10th edition (Page: 369)]
Systemic effects of inflammation:
1) Fever:
Cytokines (TNF, IL-1) stimulate the production of prostaglandins in the hypothalamus.
| Mechanism of fever: Bacterial products, LPS (Exogenous pyrogen) → stimulate leukocytes to release cytokines such as IL-1, and TNF (endogenous pyrogen) → increase the enzyme cyclooxygenase that converts AA to prostaglandins (PGE2) → stimulate the production of neurotransmitter by hypothalamus such as cyclic AMP → reset the body’s steady-state temperature to a higher level by reducing heat loss (via vasoconstriction) and increasing heat generation (through effects on brown fat & skeletal muscle). NSAIDs, including aspirin, reduce fever by inhibiting prostaglandin synthesis. |
2) Elevated level of Acute phase protein:
Acute phase proteins are plasma proteins, mostly synthesized in the liver, whose plasma concentration may increase several folds in response to inflammatory stimuli.
Example of acute phase protein:
- C-reactive protein (CRP),
- Fibrinogen,
- Serum amyloid A protein (SAA),
- Hepcidin,
- Haptoglobins,
- Ceruloplasmin,
- Complement components
Importance of acute phase protein:
- CRP and SAA act as opsonin and fix complement (bind with a microbial cell wall), bind to chromatin, and clear necrotic cell nuclei.
- Raised ESR in inflammation caused by fibrinogen. Fibrinogen binds to red cells and causes them to form stacks (rouleaux) that sediment more rapidly at unit gravity than do individual red cells.
- Prolonged production of SAA causes secondary amyloidosis in chronic inflammation.
- Hepcidin is an iron regulatory peptide responsible for anemia. Hepcidin is a small protein that reduces the availability of iron to erythroid progenitors in the bone marrow; over time, this effect may lead to the anemia of chronic inflammation
3) Leukocytosis: (↑ WBC)
- Neutrophilia: Mostly in bacterial infections
- Eosinophilia: (Allergies and helminth infestations) Bronchial asthma, hay fever, a parasitic infection.
- Lymphocytosis (↑ lymphocytes)): Viral infections
- Leucopenia (↓ in WBC): Certain infections (typhoid fever, infection caused by virus, rickettsia, protozoa)
4) Others:
- Increased pulse and blood pressure; decreased sweating, mainly because of redirection of blood flow from cutaneous to deep vascular beds, to minimize heat loss through the skin
- rigors (shivering), chills (search for warmth), anorexia, somnolence, and malaise, probably because of the actions of cytokines on brain cells.
5) In severe bacterial infection (sepsis): DIC is caused by a high level of TNF.
In severe bacterial infections (sepsis), the large amounts of bacteria and their products in the blood stimulate the
production of enormous quantities of several cytokines, notably TNF and IL-1. High blood levels of cytokines cause various clinical manifestations such as disseminated intravascular coagulation (DIC), hypotensive shock, and metabolic disturbances, including insulin resistance and hyperglycemia. This clinical triad is known as septic shock.
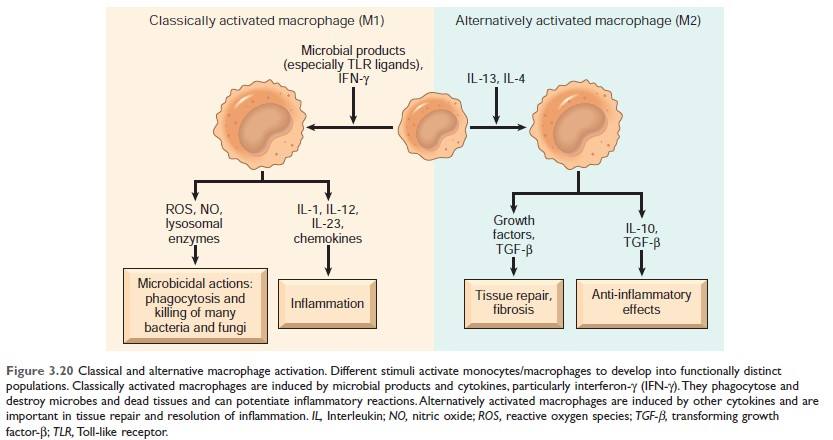
Figure: Classical and alternative macrophage activation (Source Robbin's 10th Edition)
Keypoint: Alternatively activated macrophages help in tissue repair and resolution of inflammation.
Difference between Acute inflammation and chronic inflammation
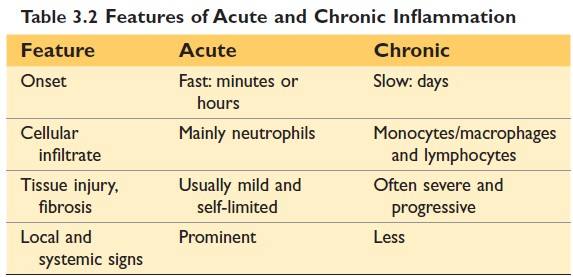
(Source Robbin's 10th Edition)
Examples of Inflammation
| Site | Inflammation | Site | Inflammation |
| Brain | Encephalitis | Joint | Arthritis |
| Meninges | Meningitis | Subcutaneous tissue | Cellulitis |
| Nerve | Neuritis | Blood vessel | Vasculitis |
| Conjunctiva | Conjunctivitis | Artery | Arteritis |
| Cornea | Keratitis | Vein | Phlebitis |
| Uveal tract | Uveitis | Lymph node | Lymphadenitis |
| Nose | Rhinitis | Lymphatic vessels | Lymphangitis |
| Oral cavity | Stomatitis | Appendix | Appendicitis |
| Salivary gland | Sialadenitis | Cecum | Typhlitis |
| Tonsil | Tonsillitis | Rectum | Proctitis |
| Larynx | Laryngitis | Breast | Mastitis |
| Pharynx | Pharyngitis | Testis | Orchitis |
| Lung | Pneumonia | Ovary | Oophoritis |
| Pleura | Pleurisy | Fallopian tube | Salpingitis |
| Liver | Hepatitis | Prostate | Prostatitis |
| Gall bladder | Cholecystitis | Uterus | Endometritis |
| Muscle | Myositis | Urinary Bladder | Cystitis |

Comments (0)