Blood (Viva)
Q.1 What are the main functions of blood?
- As a carrier of oxygen.
- As a carrier of metabolic wastes of the body of the kidney, lungs, skin, and intestine for removal.
- In the maintenance of acid-base balance.
- In the maintenance of body temperature.
- In transporting food materials to the tissues.
- In regulating water balance.
Q.2 What are the compositions of blood?
- Cellular fraction. This fraction contains erythrocytes, leukocytes, and platelets.
- Plasma fraction.
Q.3 What is the pH of blood?
7.4.
Q.4 What is the average volume of blood in the body?
5-7% of the bodyweight
Q.5 What is the specific gravity of blood?
1.054-1.060
Q.6 Name the steps involved in blood clotting.
Prothrombin Thromboplastin, Ca++ >> Thrombin
Q.7 Name the pathways of blood coagulation.
- Intrinsic pathway.
- Extrinsic pathway.
Q.8 Name the substance of the blood clot.
Fibrin
Q.9 Name the inhibitors of blood coagulation.
Oxalates, citrates, heparin
Q.10 What are the factors which delay blood coagulation?
- Low level of prothrombin.
- Deficiency of vitamin C.
- Obstruction of bile duct.
Q. Name the blood buffers?
| BHCO3 H2CO3 Bicarbonate Buffer |
| B2HPO4 B H2PO4 Phosphate Buffer |
| B Proteinate H Protein Protein Buffer |
| B.Hb H.Hb Haemoglobin Bufffer |
| B.HbO2 H.HbO2 Oxyhaemoglobin Buffer |
Q.12 What is serum?
The blood on clotting without any anticoagulant, gives a clear supernatant called serum.
Q.13 What is plasma?
Plasma is obtained from blood which is prevented from clotting.
Q.14 What is the difference between serum and plasma?
Serum differs from plasma, in that it contains no fibrinogen.
Plasma–Fibrinogen = Serum
Q.15 What is normal hemoglobin level in blood?
10-15 gm%.
Q.16 Hemoglobin is measured by which method?
Sahli method
Q.17 Where is hemoglobin present?
Hemoglobin is present in RBC.
Q.18 Hemoglobin belong to which class of proteins?
It belongs to a class of conjugated protein. Hemoglobin = Heme + globin.
Q.20 What is the prosthetic group of hemoglobin?
Heme
Q.21 What is heme?
Heme is ferrous protoporphyrin.
Q.22 What is the state of iron in hemoglobin?
Iron is present in the ferrous state (i.e. + 2 state).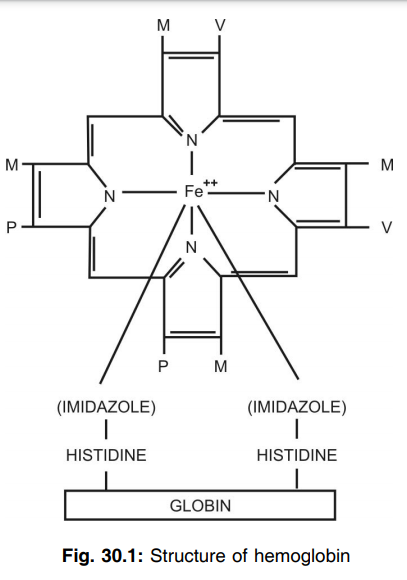
Q.23 What is the state of iron in oxyhemoglobin?
Ferrous (+ 2 state)
Q.24 Which amino acid is responsible for the buffering action of hemoglobin?
Histidine.
Q.25 What are the starting materials of hemoglobin synthesis?
Succinyl CoA and glycine.
Q.26 What is porphyria?
Accumulation of coproporphyrin and uroporphyrin in the blood with the excessive elimination in urine and feces is called porphyria.
Q.27 What is methemoglobin?
In methemoglobin, iron is in the ferric state.
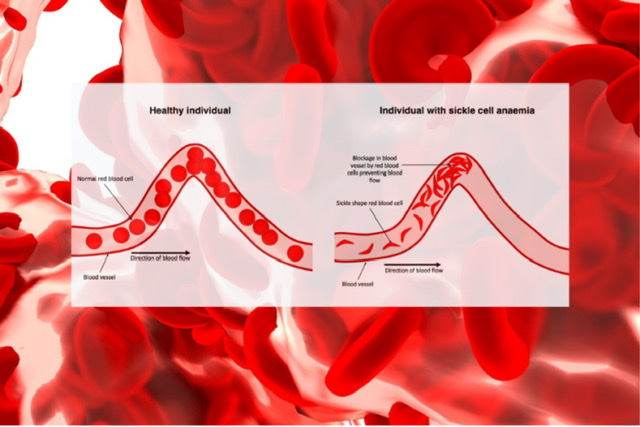
Q.28 What is sickle cell anemia?
In the normal hemoglobin, if the glutamic acid is replaced by valine at 6 position in the b-chain, sickle cell anemia results.
Q.29 Persons with sickle cell anemia are having which advantage?
Plasmodium falciparum cannot invade sickled RBC so, the person heterogenous for sickle cell anemia escape from the deadly disease.
Q.30 What are the various types of hemoglobin? Give important differences between them.
| Chains | |
| HbA | - A1 (98%) 2α 2β - A2 (2%) 2α 2δ |
| HbF | 2α 2γ |
| HbS | glu→val at 6 position 2α 2β |
| HbH | 4β |
Q.31 What are thalassemias?
Mutations of regulator genes may sometimes represses the synthesis of one type of polypeptide chain of globin, with a compensatory increase in the synthesis of other types of polypeptide chains of globin. The latter may replace the repressed polypeptide chains in the globin molecules thus producing abnormal hemoglobin. Such genetic mutations are called thalassemias.
Q.32 Which mutation is present in thalassemia?
Frameshift mutation
Q.33 Give inhibitor of vitamin K which acts as an anticoagulant.
Dicumarol, i.e.warfarin.
Q.34 What is the shape of the O2-Hbdissociation curve?
Sigmoidal
Q.35 What is the shape of the oxygen myoglobin dissolution curve?
Rectangular hyperbola.
Q.36 In lead poisoning RBCs are termed as:
Cabot’s ring Howell-Jolly bodies.
Q.37 Which problem arises by massive blood transfusion?
Hemosiderosis
Q.38 Which problem arises by long-standing blood transfusion?
Since 2,3-DPG level decreases in long time stored blood. So, the affinity of O2 towards Hb increase. So, a person receiving blood cannot get O2 adequately.
Q.39 Which Hb is having the highest affinity for O2 and why?
HbF (Fetal hemoglobin) because of low 2,3- DPG level.
Q.40 If all four chains of Hb are b type, then the condition is known as?
Hydrops fetalis.
Q.41 Name an Rh incompatibility disease.
Erythroblastosis fetalis.
Q.42 This condition arises in which circumstances?
This condition arises when Rh +ve male is married to Rh –ve female.
Q.43 Define Rh factor.
Presence or absence of protein is referred to as the Rh factor.
Q.44 What are different type of Rh factor?
There are 2 types:
1. Rh + ve
2. Rh – ve.
In Rh +ve if your blood contains the protein then it is called Rh +ve. and If your blood does not contain the protein your blood is said to be Rh – ve.
Q.45 What are the functions of Blood?
The main function of blood:
- It supplies nutrients such as oxygen, glucose to tissues.
- It supplies constitutional elements to tissues.
- It removes waste products such as carbon dioxide and lactic acid.
- t maintains body temperature.
- Controls pH.
- Removes toxins from the body.
- It helps in regulating body fluid electrolytes.
Also read: Blood Notes
Also read: Biochemistry Questions & Answers

Comments (0)