Biological Oxidation (Viva)
Q.1 What is oxidation?
Oxidation is defined as the loss of electrons
Fe++ → Fe+++
or
removal of hydrogen
C2H5OH → CH3CHO
or
addition of oxygen
CH3CHO → CH3 COOH
Q.2 What do you mean by biological oxidation?
The stepwise degradation of metabolites for the liberation of energy carried out in the system.
Q.3 Give Gibbs free energy equation.
| ΔG = ΔH—TΔ S |
| ΔH = enthalpy of reaction T = temp (K) ΔS = entropy change. |
Q.4 What inference is drawn from this reaction?
If ΔG is +ve then the reaction is endothermic while -ve implies, reaction to be exogenic.
Q.5. Who gives the concept of a high energy phosphate bond?
Lippmann introduced the concept of a high energy phosphate bond.
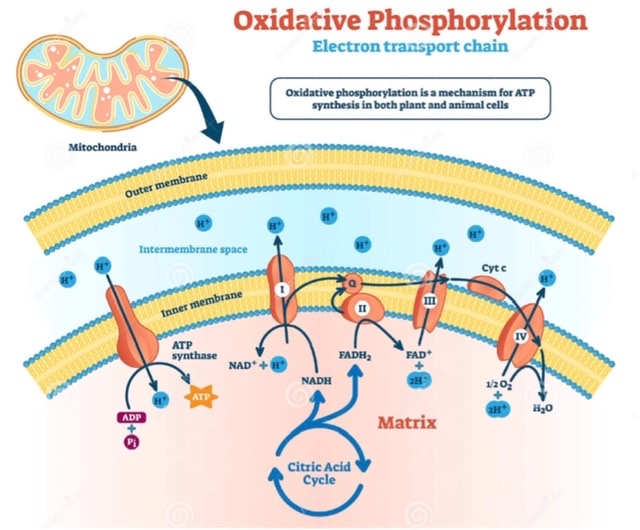
Q.6 Name the components of the respiratory chain.
NAD+→ Fp→CoQ→Cyt b→Cyt C1→ Cyt c→Cyt a → Cyt a3
Q.7 In which order the components of the respiratory chain are arranged?
The components of the respiratory chain are arranged in order of increasing redox potential. NAD+ has the lowest redox potential whereas Cyt a3 has the highest redox potential in the respiratory chain.
Q.8 Name the various ATP sites in the respiratory chain.
- NAD+ to FAD.
- Cyt b to Cyt C1
- Cyt a to Cyt a3
Q.9 What should be the minimum redox potential difference for the generation of ATP?
0.2 volts
Q.10 What is the ATP yield in the respiratory chain?
32 molecules of ATP (28 + 2 + 2)
Q.11 Which part of the NAD+ accepts electrons?
Nicotinamide
Q.12 Which part of the FAD accepts electrons?
Flavin
Q.13 What is oxidative phosphorylation?
Oxidative phosphorylation is the process in which ATP is formed as electrons are transferred from NADH or FADH2 to oxygen by a series of electron carriers.
Q.14 Name the inhibitors of various energy sites in the respiratory chain?
Inhibitors of site I = rotenone, amobarbital, piericidin.
Inhibitors of site II = Antimycin, BAL
Inhibitors of site III = H2S, CO2, CN¯.
Q.15 What is substrate-level phosphorylation?
In substrate-level phosphorylation, high energy phosphate bond formation takes place on the substrate without undergoing into the respiratory chain.
Q.16 Give examples of substrate-level phosphorylation.
D-Glyceraldehyde-3PO4 → 3 Phosphoglyceric acid.
Phosphoenol pyruvic acid → Pyruvate Succinyl CoA → Succinic acid.
Q.17 What is the P/O ratio?
P/O ratio is defined as the number of inorganic phosphate taken to phosphorylate ADP per atom of oxygen consumed.
P/ Q = No. of inorganic phosphate to phosphorylate ADP /No. of atom of oxygen consumed
Q.18 Name a few reactions where the P/O ratio is 2.
- Succinate ——→ Malate (TCA cycle)
- Acyl CoA ——→ β unsat. Acyl CoA (β-oxidation of fatty acids).
Q.19 Name a few high energy compounds.
Adenosine triphosphate, adenosine diphosphate, Ionosine triphosphate, etc.
Q.20 What are uncouplers?
Uncoupler are those substances which separate oxidation from phosphorylation,
e.g. Dinitro phenol.
Q.21 Name the different classes of oxidoreductases.
- Oxidases.
- Dehydrogenases.
- Hydroperoxidases.
- Oxygenases
Q.22 Give some examples of oxidases.
- Cytochrome oxidase.
- FMN, FAD.
- Xanthine oxidase.
- Glucose oxidase.
- L-amino acid oxidase.
Q.23 Which enzyme is responsible for the lowering of superoxide ion?
Superoxide dismutase (SOD)
Q.24 Superoxide dismutase (SOD) contains which ions?
Superoxide dismutase enzymes is of two types:
- Up to plasmic SOD.
- Mitochondrial SOD.
Up to plasmic SOD contains copper and uric ions whereas mitochondrial SOD contains manganese.
Q.25 Who proposed the chemiosmotic theory?
Peter Mitchelle was the person to propose the chemiosmotic theory.
Q.26 Name the inhibitor of adenine nucleotide transporter.
Atractyioside is an inhibitor of adenine, nucleotide transporter.
Also Read: Biochemistry Questions & Answers

Comments (0)