Biophysics (VIva)
Q.1 What is pH?
pH is defined as the negative logarithm of the hydrogen ion concentration.
pH = – log [H+]
or pH= 1
log [H+]
Q.2 What is the relationship between H+ ions and pH?
There is an inverse relationship between the two. As the H+ ion concentration increases and vice versa.
Q.3 What are the methods by which pH can be determined?
pH can be determined by:
- Indicators.
- pH paper.
- Buffers.
- pH meter
Q.4 What is the pH of distilled water, gastric juice, intestinal juice, pancreatic juice, blood, and urine?
- Distilled water pH = 7.
- Gastric juice pH = 0.9-1.
- Intestinal juice pH = 7-8.
- Pancreatic juice pH = 7.5-8.
- Blood pH = 7.4
- Urine pH = 5.5.- 6.5
Q.5 What is the pH of each of the following solutions?
i. 10-3 N HCl
ii. 10-2 N NaOH
i.pH - 3
ii. pH -12
Q.6 What are buffers?
Buffers are solutions that resist changes in their pH when a small amount of acids or alkalies are added to them. Buffers act like shock absorber against the sudden changes of pH.
Q.7 What is the composition of a buffer?
Buffer is a pair of a weak acid and its salt with a strong base.
Example: CH3COOH/CH3COONa
Q.8 Give a few examples of commonly used buffers in the laboratory.
• Acetate buffer (Sodium acetate/Acetic acid)
• Phosphate citrate buffer (Na2HPO4 KH2PO4)
• Citrate buffer (Sodium citrate/Citric acid)
• Barbitone buffer (Sodium diethyl barbiturate/Diethyl barbituric acid).
Q.9 How the pH of a buffer solution can be calculated?
pH of a buffer solution can be calculated by the Henderson Hasselbalch equation.
pH = pK + log [Conc. of salt/Conc. of acid]
Q.10 What is pK?
pK is the pH at which the acid is half neutralized.
Q.11 Name the various body buffers.
Buffers operating in the body are:
- H2CO3/BHCO3.
- H.protein/B.protein.
- BH2PO4/B2HPO4
- HHb/BHb.
- HHbO2/BHbO2.
- H. organic acid/B. organic acid
Q.12 What is the importance of buffers in the body?
Buffers maintain the pH of the various fluids of the body compartments constant despite wide variation in the H+ ion concentration which is produced in the normal course of the metabolism of the body as a by-product which otherwise could have lowered down the pH.
1. To regulate the pH of the body fluids.
2. To control the pH in chemical reactions catalyzed by enzymes.
Q.13 Name some pH disorders.
If the pH of the body decreases, it is referred as to acidosis, and if increases it is referred to as alkalosis. The cause of both may be metabolic or respiratory.
Q.14 What is diffusion?
It is the movement of particles from their higher concentration to lower concentration.
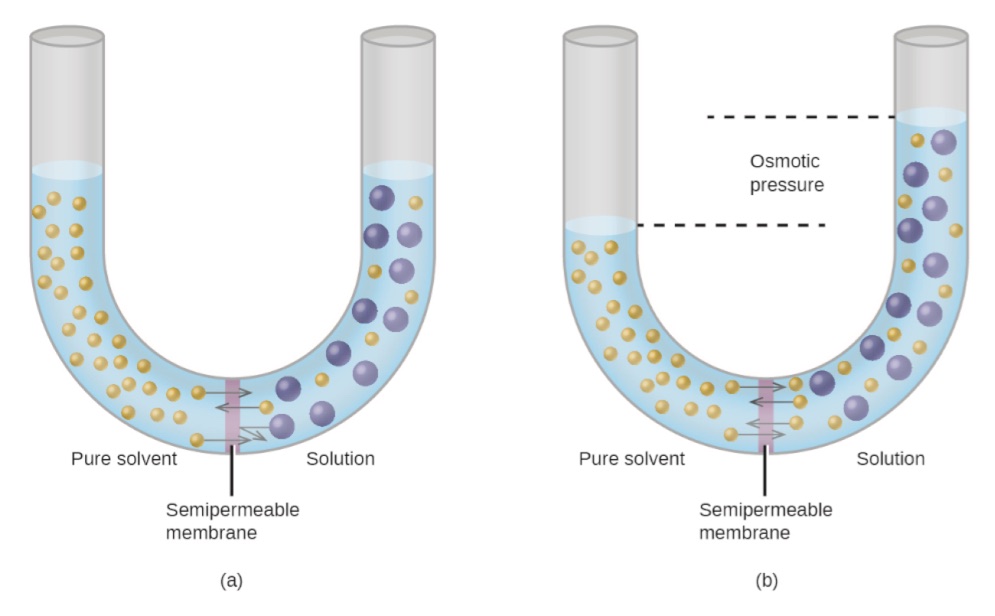
Q.15 What is osmosis?
The movement of solvent molecules from a pure solvent to dilute solution through a semipermeable membrane is called osmosis.
Q.16 What is a semipermeable membrane?
A membrane that allows the solvent molecule to pass but does not allow the passage of the solute molecule.
Q.17 What is osmotic pressure?
It is the pressure generated by osmosis. When solvent moves from a concentrated solution to a dilute solution through a semipermeable membrane, then particles of solvent exert a pressure on the semipermeable membrane, it is called osmotic pressure.
Q.18 What are the factors on which the osmotic pressure depends?
Osmotic pressure depends only on the number of dissolved particles and is independent of the size of the particles.
Q.19 Why an ionized solution has more osmotic pressure than an unionized solution?
The ionized solution gives rise to more number of particles or ions on ionization and each ion will exert an osmotic pressure; hence, the osmotic pressure is more whereas, in unionized solution, the number of molecules remains the same.
Q.20 Which will have greater osmotic pressure?
a. 1 molar solution of NaCl.
1 molar solution of CaCl2.
1 molar solution of glucose.
b. What will be the order of osmotic pressure?
a. 1 molar solution of CaCl2 will have the maximum osmotic pressure because CaCl2 on ionization gives rise to 3 ions and each ion will exert an independent osmotic pressure. Whereas NaCl gives two ions on ionization and glucose does not ionize.
b. The order of osmotic pressure is 1 molar solution of CaCl2 > 1 molar solution of NaCl > 1 molar solution of glucose.
Q.21 What is Gibbs-Donnan equilibrium?
The unequal distribution of diffusible ions across the membrane when a non-diffusible ion is present on one side of a membrane leads to Gibbs-Donnan equilibrium.
Q.22 Explain the importance of GibbsDonnan equilibrium.
1. In the maintenance of differential concentrations between the various compartments of the body.
2. In the process of absorption.
3. In the process of secretion.
Q.23 Explain the following in terms of osmotic pressure.
1. Isotonic solution?
2. Hypertonic solution?
3. Hypotonic solution?
• Isotonic solution: They have the same osmotic pressure as within the cells.
• Hypertonic solution: They have higher osmotic pressure than within the cells.
• Hypotonic solution: They have lower osmotic pressure than within the cells.
Q.24 What is surface tension?
The force by which the surface molecules are held together and form a membrane over the surface of the liquid is called surface tension.
Q.25 Name the substance which lowers down the surface tension.
Bile salts, organic substances, ammonia, strong mineral acids.
Q.26 What is adsorption?
The process of holding up of substances from the solution on the surface is called adsorption.
Q.27 What is hydrotrophy?
The process whereby water-insoluble substances are made soluble without undergoing any chemical change.
Q.28 What are crystalloids?
Crystalloids are those substances that diffuse readily through membranes.
Q.29 What are colloids?
Colloids are those substances which do not diffuse through membranes.
Q.30 What are protective colloids?
Colloids that prevents other substances from being precipitated are called protective colloids.
Q.31 What are the roles played by protective colloids?
Protective colloids play an important role in physiologically.
1. Proteins of milk serve as protective colloids to calcium phosphate present in the milk.
2. Blood proteins serve as protective colloids to the calcium phosphate of the blood.
Q.32 What is dialysis?
The process of separating crystalloids from colloids by diffusion through a membrane by osmotic force is called dialysis.
Q.33 What are the indicators?
Indicators are substances that change in color with a change in pH of solutions in which they are present. They behave like weak acids or weak bases, the ionized and unionized forms of which differ in color.
Q.34 What is the nature of indicators?
Indicators are dyes which are weak organic acids or weak organic bases and have the property of dissociating.
Q.35 What are the common indicators generally used? Give their effective pH range and their acid and alkaline color.
| Indicators | pH range | Acid color | Alkaline color |
| Thymol blue (acid range) | 1.2-2.8 | Red | Yellow |
| Methyl yellow | 2.9-4.0 | Red | Yellow |
| Methyl orange (Topfers indicators) | 3.1-4.4 | Red | Yellow-orange |
| Methyl red | 4.3-6.1 | Red | Yellow |
| Phenol red | 6.7-8.3 | Yellow | Red |
| Thymol blue (alkaline range) | 8.0-9.6 | Yellow | Blue |
| Phenolphthalein | 8.2-10 | Colorless | Red |
Q.36 What is milliequivalent?
Milliequivalent is one-thousandth of a gram equivalent weight.
Q.37 What is normality?
Normality is the number of equivalents of solute in one liter of solution.
Q.38 What is molarity?
Molarity is the number of moles of solute in one liter of solution.
Q.39 What is osmolarity?
Osmolarity is the same as molarity except for the fact that in osmolarity only osmotically active particles are considered.
Q.40 What is molality?
The molality of a solution refers to the number of moles of solute in 1000 gm of solvent.
Q.41 What is the relationship between milligram percent and milliequivalent?
milliequivalent/liter = milligram percent × 10 × valency
molecular weight or atomic weight
Q.42 What is the milligram percent of calcium when its concentration is 5 mEq/ L?
mg% × 10 × 2 = 10 mg%
40
Q.43 What is the milliequivalent per liter of sodium when its concentration is 322-milligram percent.
322 ×10 × 1 = 140 mEq/L.
23
Q.44 What is chromatography?
Chromatography is defined as the analytical technique used for separating mixtures on the basis of difference in affinity for a stationary and a mobile phase.
Q.45 What are the various types of chromatography?
1. Thin-layer chromatography.
2. Column chromatography.
3. Paper chromatography.
4. Gas chromatography.
Q.46 What kind of paper is required for paper chromatography?
We use chromatographic strip or Whatman filter paper 1 for paper chromatography.
Q.47 What are the units for expressing chromatography?
Rf
Q.48 What is Rf?
Rf is defined as the ratio of distance traveled by the compound to the distance traveled by the solvent.
Q.49 What is the partition coefficient?
The partition coefficient is the ratio of the concentration of solute in phase-1 to that of other phases.
Q.50 How partition coefficient is designated?
Kd
Q.51 What is the significance of the partition coefficient?
The position to which a given solute moves up the paper is largely dependent on its partition coefficient at a given temperature.
Q.52 What are the uses of chromatography?
1. Separation of amino acid in patients with inborn errors of metabolism.
2. Identification of reducing sugars in the serum.
3. Separation of drug metabolites.
4. For the detection of poisons.
Also read: Biochemistry Questions & Answers

Comments (0)