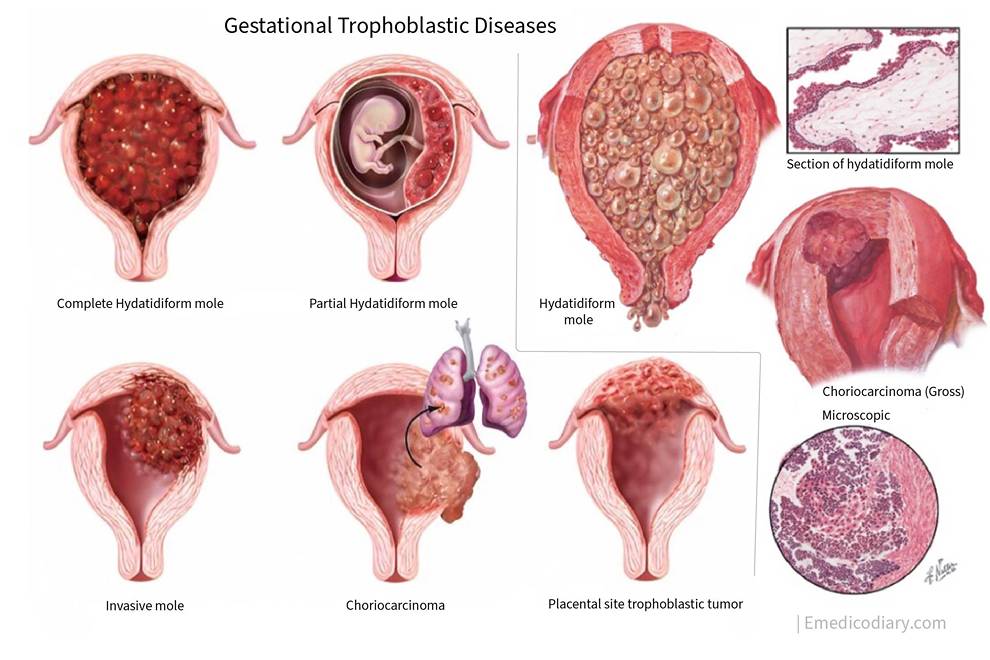
Gestational Trophoblastic Disease
The gestational trophoblastic disease is the spectrum of proliferative abnormalities of trophoblasts that are associated with pregnancy. Persistent gestational trophoblastic disease is referred to as gestational trophoblastic neoplasia.

Classification of gestational trophoblastic disease
A. Conventional
- Hydatidiform mole
- Invasive mole
- Choriocarcinoma
- Placental site trophoblastic tumor
B. Modified WHO classification of gestational trophoblastic disease (1998)
1. Hydatidiform mole:
- Complete mole
- Partial mole
2. Invasive mole
3. Placental site trophoblastic disease
4. Choriocarcinoma
- Nonmetastatic disease
- Metastatic disease
Hydatidiform mole
Hydatidiform mole is an abnormal condition of the placenta where there are partly degenerative and partly proliferative changes in the young chorionic villi. The Hydatidiform mole is a benign neoplasm of chorion with malignant potential. It is the most common form of gestational trophoblastic disease. Hydatidiform mole is also known as a molar pregnancy.
Histology of Hydatidiform mole:
Gross finding of hydatidiform mole:
Hydatidiform mole is characterized by multiple grape-like vesicles filling and distending the uterus. The vesicles are filled with interstitial fluid similar to ascitic or edema fluid but rich in β hCG. Usually, the fetus is absent.
Microscopic finding of Hydatidiform mole:
- The proliferation of syncytiotrophoblasts and cytotrophoblastic epithelium marks hydatidiform mole.
- There is thinning of the stromal tissue due to hydropic degeneration.
- Absence of blood vessels
- Maintenance of villous pattern
Incidence of Hydatidiform mole
The incidence of molar pregnancy is maximum in Asia and South America and least in Us. The maximum incidence is in the Philippines (1 in 80). In India, it is 1 in 400 pregnancies.
Risk factors of Hydatidiform mole:
- More in women too elderly (>35 yrs) or too younger(<18 yrs)
- Previous history of molar pregnancy
- Disturbed maternal immune mechanism
- Nutrient deficient: Diet deficient in protein, folic acid, and vitamin A.
- Cytogenetic abnormality: Complete moles have a 46XX karyotype (85%) i.e. chromosomes are derived entirely from the father. When sperm fertilized the empty ovum, the chromosomes of sperm duplicate after meiosis.
Maternal age of more than 35 years and dietary deficiencies are risk factors for complete mole whereas partial mole is linked to the use of oral contraceptive pills and a history of irregular menstruation.
Partial mole:
A partial mole occurs when two sperm fertilize an egg. So, the karyotype of the partial mole is triploid .i.e the chromosome number is 69 (69XXY or 69XXX). The extra haploid set of chromosomes usually is derived from the father.
Microscopic finding of Partial mole
- Trophoblastic proliferation is less marked.
- Some blood vessels are present.
- Some fetal tissues are present.
Histopathological examination of Partial mole:
- Trophoblastic inclusion body present.
- Scalloping of chorionic villi present.
Clinical features of Partial mole:
The most common presenting feature of the partial mole is bleeding per vagina following a period of amenorrhea. The history of the passage of grape-like vesicles is less frequent in a partial mole.
On Per abdominal Examination, the height of the uterus is equal to or less than the period of gestation.
The investigation of choice of diagnosis of hydatidiform mole is USG. On USG partial mole is often confused with missed abortion. The gold standard for diagnosis of the mole is the histopathological examination.
The level of β hCG levels in a partial mole is raised but not markedly raised as that of a complete mole. Theca lutein cyst is not seen in the ovary. The chances of persistent gestational trophoblastic disease or gestational trophoblastic neoplasia in the partial mole is 3-5% and the risk of choriocarcinoma is<1%.
COMPLETE MOLE:
Complete mole occurs when a sperm fertilized an egg that has lost its chromosome (empty ovum). Thus, chromosomes are derived entirely from the father. When sperm fertilized the empty ovum, the chromosomes of sperm duplicate after meiosis. So the karyotype of the complete mole is 46XX (in 90% of cases) and in 10% of cases(46XY or 45X0)
Microscopic finding of Complete mole
- Extensive trophoblastic proliferation
- No blood vessels
- No fetal tissue
Histopathological examination of Complete mole
- Trophoblastic inclusion body absent
- Scalloping of chorionic villi absent
Clinical feature of Complete mole
The most common presenting feature of the complete mole is bleeding per vagina following the short period of amenorrhea (8 - 12 weeks). It is also associated with Lower abdominal pain. The history of the passage of grape-like vesicles per vagina is the diagnostic feature of hydatidiform mole.
Other constitutional symptoms of the complete mole are:
- Thyrotoxicosis
- Pre-eclampsia
- Hyperemesis gravidarum
On per abdominal examination, there is a doughy feeling (firm elastic) of the uterus (due to the absence of amniotic fluid) and the height of the uterus is found to be more than the gestation period in about 70% of cases.
On per vaginal examination, vesicles can be found. Enlargement of the ovary (Theca lutein cysts) may be palpable.
Investigations done in Hydatidiform mole are:
- CBC
- Blood grouping & Rh typing
- Liver function test
- Renal function test
- Thyroid function test
- USG of the uterus: It shows snow storm-like appearance
- Serum beta HCG markedly increased (above 100,000 mIU/ml)
- Chest x-ray
About 15–20% of complete moles have a chance to progress into Persistent Gestational Trophoblastic Disease. In about 1-4% of cases, there is a risk of recurrence of hydatidiform mole in future pregnancy. The risk of choriocarcinoma from a hydatidiform mole is about 2 - 10 %.
Management of hydatidiform mole
Spontaneous expulsion occurs at around 16 weeks and is rarely delayed beyond 28 weeks. The treatment of choice in a hydatidiform mole is suction evacuation and curettage irrespective of uterine size. Suction evacuation is done first. After most of the molar tissue has been removed by aspiration, oxytocin is given. Once myometrium is contracted, thorough but gentle curettage with a large sharp curette (10-12 mm)is performed. An intraoperative ultrasonographic examination may help document that the uterine cavity has been emptied.
Hysterectomy is indicated only in the case of:
- Female has completed her family irrespective of age
- Age of patient >35 years
- Uncontrolled hemorrhage or perforation of the uterus during suction evacuation.
Hysterectomy decreases the risk of GTN by 5 folds. But it is rarely done these days.
Follow up In Hydatidiform mole
Routine follow-up is mandatory for all cases for at least 6 months following molar pregnancy/hydatidiform mole. Doing a hysterectomy does not negate the need for follow-up. First beta hCG is obtained 48 hrs after evacuation. Then monitor serum hCG levels every week till they become normal for three consecutive weeks. Once the hCG levels fall to a normal level for 3 weeks, test the patient monthly for 6 months; then follow-up is discontinued and pregnancy is allowed.
During the 6 month surveillance period, the patient is advised not to become pregnant because during this 6 month period, new pregnancy increases the level of beta hCG further which increases the chance of conversion of molar pregnancy into gestational trophoblastic neoplasia.
In a partial mole, the time for β hCG level to come to normal is 7 weeks & for a complete mole is around 9 weeks.
Prophylactic chemotherapy:
Routine prophylactic chemotherapy is not advocated nowadays after the evacuation of hydatidiform mole/molar pregnancy.
Indications of prophylactic chemotherapy:
- High hCG level >10^5IU/L
- Increase the size of the uterus more than gestational age.
- Age more or equal to 40 yrs.
- Bilateral theca cyst more or equal to 6 cm
The drug of choice in molar pregnancy is Methotrexate.
Contraceptive advice:
Estrogen-progestin contraceptives or depot medroxyprogesterone are usually used to prevent a subsequent pregnancy during the period of surveillance. The contraceptive of choice is combined oral pills. (Earlier contraceptive of choice was the barrier method)
GESTATIONAL TROPHOBLASTIC NEOPLASIA (GTN)
Gestational trophoblastic neoplasia includes invasive mole, choriocarcinoma, placental site trophoblastic tumor, and epithelial trophoblastic tumor. Gestational trophoblastic neoplasia almost always develops with or follows some form of pregnancy.
Among all the cases of choriocarcinoma,
• 50% develop following a hydatiform mole.
• 25% develop the following abortion
• 20% develop following a full-term pregnancy
• 5% develop following an ectopic pregnancy.
Note:
The most common Gestational trophoblastic neoplasia is Invasive mole. The second most common Gestational trophoblastic neoplasia is Choriocarcinoma. The rare Gestational trophoblastic neoplasia is a Placental site trophoblastic tumor.
The most common Gestational trophoblastic neoplasia after a molar pregnancy is Invasive mole. The most common Gestational trophoblastic neoplasia after non-molar pregnancy: choriocarcinoma. Choriocarcinoma mostly occurs after molar pregnancy.
Risk factors of Gestational trophoblastic neoplasia are
- Age of female > 40 yrs
- beta - hCG levels > 10^6 mIU/ml
- Uterine size larger than gestational age
- Bilateral theca lutein cyst >6cm
- Slow decline in beta hCG levels.
Criteria for diagnosis of post-molar gestational trophoblastic neoplasia are
- When 4 consecutive hCG values are plateau D1, D7, D14, D21
- 3 consecutive hCG values are rising (>10% of previous value) D1, D7, D14.
- Beta hCG level remains above normal even after 6 months of evacuation
- Histological criteria for choriocarcinoma
Clinical features of Gestational trophoblastic neoplasia are
- Patients present with continuous bleeding P/V even after evacuation
- Uterine sub involution
- Invasive moles can lead to myometrium perforation and intraperitoneal hemorrhage.
- Persistence of theca lutein cysts.
- Metastasis: In choriocarcinoma, the most common site of metastasis is the lungs.
CHORIOCARCINOMA
Choriocarcinoma is the most malignant tumor of the uterus. The most common mode of spread of choriocarcinoma is hematogenous.
The most common sites in choriocarcinoma are the lungs (80%), pelvis(20%), liver(10%), and brain (10%).
Symptoms of choriocarcinoma
Mostly presents as irregular bleeding or uterine hemorrhage following an abortion, molar pregnancy, or normal delivery.
Metastasis of choriocarcinoma
Lung metastasis of choriocarcinoma
Lung metastasis of choriocarcinoma is seen in 80% of cases. Patients of choriocarcinoma with Lung metastasis present with respiratory symptoms like dyspnoea, hemoptysis, chest pain, etc. On X-ray, it may produce the following four patterns:
- an alveolar snowstorm appearance
- discrete rounded densities or canon ball appearance
- Pleural effusion
Vaginal metastasis of choriocarcinoma
Vaginal metastasis of choriocarcinoma is seen in 30% of cases. Metastasis occurs in sub urethra or in fornices, Metastasis appears as purple hemorrhagic projections which are highly vascular and bleed on touch.
Tumor markers
The tumor marker for choriocarcinoma is beta hCG. The Tumor marker for placental site trophoblastic tumor is hPL.
Staging of GTN
FIGO anatomic staging of gestational trophoblastic neoplasia is
- Stage 1: The lesion is confined to the uterus
- stage 2: The lesion spreads outside the uterus but within the pelvis
- Stage 3: The lesion metastasizes to the lungs
- Stage 4: The lesion metastasizes to sites such as the brain, liver, or gastrointestinal tract.
WHO scoring of gestational trophoblastic neoplasia
Good prognosis / low risk of gestational trophoblastic neoplasia are:
- Age less or equal to 39 years.
- Type of antecedent pregnancy: molar pregnancy
- Duration of antecedent pregnancy<4 months duration.
- Initial serum hCG level > 40,000 mIU/ml
- Metastasis limited to the lung and vagina
- Metastasis number <4
- Size of tumor: <3cm
- Antecedent chemotherapy - single-agent chemotherapy
(Total score <=6)
Poor prognosis / high risks of gestational trophoblastic neoplasia are:
- Age >= 40 years
- Duration of antecedent pregnancy>12 months.
- Initial serum hCG >40,000 mIU/ml
- Brain or liver metastasis
- Failure of prior chemotherapy
- Type of antecedent pregnancy: full-term pregnancy
- Metastasis number: >8
- Size of tumor: >5cm
- Antecedent chemotherapy: multiple agent chemotherapies
(Total score >=7)
Management of gestational trophoblastic neoplasia is GTNs are very chemotherapy-sensitive tumors, so the treatment of choice for gestational trophoblastic neoplasia is chemotherapy except; for placental site trophoblastic tumor.
For Low-risk GTN:
- Single drug therapy (Methotrexate)
- Actinomycin D (in resistant case)
For high-risk GTN: Multidrug therapy
- Etoposide
- Methotrexate
- Actinomycin D
- Cyclophosphamide
- Oncovin (O vincristine)
Follow-up of gestational trophoblastic neoplasia:
Follow-up is mandatory for all patients. For low-risk patients, the period of surveillance is 12 months, and for high-risk patients for 24 months. During follow-up, serum beta-hCG is measured weekly until it is negative for three consecutive weeks. Thereafter it is measured monthly for 6 months and then 6 months.
Placental site trophoblastic tumor
Placental site trophoblastic tumor arises from the intermediate trophoblasts of the placental bed and is composed mainly of cytotrophoblastic cells. The most common site of placental site trophoblastic tumor is the posterior wall of the uterus near the fundus. The tumor most commonly occurs after a full-term pregnancy.
Placental site trophoblastic tumors have been associated with modestly elevated serum beta-hCG levels but they produce variant forms of beta - hCG. Human placental lactogen (hPL) is also a tumor marker for these tumors. The patient presents with vaginal bleeding. Local invasion into myometrium and lymphatics occurs.
Placental site trophoblastic tumor is not responsive to chemotherapy. Hysterectomy is the preferred treatment in case of placental site trophoblastic tumor
Indication for surgery in gestational trophoblastic neoplasia (GTN)
- Placental site trophoblastic tumor
- Invasive mole which perforates the uterus
- Hydatiform mole >40 years and family is complete.
Indication for radiotherapy in gestational trophoblastic neoplasia (GTN)
1. Brain and liver metastasis in choriocarcinoma
The recurrence rate of gestational trophoblastic disease (GTD) is 2%.
In the next pregnancy:
- fertility won't be impaired
- next pregnancy outcome will be normal.
- the rate of congenital malformation is not increased.
After delivery of the next pregnancy:
1. send placenta or product of conception for histopathology
2. check beta hCG level 6 weeks postpartum

Comments (0)